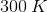All College Chemistry Resources
Example Questions
Example Question #1 : Thermodynamics
The second law of thermodynamics states which of the following is true regarding an isolated system?
The entropy can only decrease
Energy in the form of heat is always conserved
The entropy approaches a constant value as the temperature of the system approaches zero Kelvin
Two systems in thermal equilibrium with a third system are in thermal equilibrium with each other
The entropy can only increase
The entropy can only increase
The entropy cannot decrease in an isolated system because the energy can only be degraded. Since the system is isolated, no higher-grade energy—or any energy at all—is being introduced into the system. As a result, the entropy cannot decrease. The other answer choices relate to the other laws of thermodynamics.
Example Question #1 : Laws Of Thermodynamics
Which of the following statements is true of standard states?
Solute of 
Solute in 
Gas at
Gas at 
Temperature at
Gas at 
Standard states are defined as a specific set of conditions, such as when a gas is at 


Standard enthalpy of formation, the energy required for form 1 mole of a compound from its constituent elements, occurs when elements are in their standard states.
Example Question #1 : Thermodynamics
How much heat is required to raise the temperature of 




Example Question #3 : Thermodynamics
How much heat is required to raise the temperature of 


Example Question #2 : Laws Of Thermodynamics
"In a natural thermodynamic process, the sum of the entropies of the interacting systems increases." Which law of thermodynamics does this statement refer to?
Third law of thermodynamics
First law of thermodynamics
Zeroth law of thermodynamics
Second law of thermodynamics
Second law of thermodynamics
There are four main laws of thermodynamics, which describe how temperature, energy, and entropy behave under various circumstances. The zeroth law of thermodynamics helps to define temperature; it states that if two systems are each in thermal equilibrium with a third system, they must be in thermal equilibrium with each other. The first law of thermodynamics negates the possibility of perpetual motion; it states that when energy passes into or out of a system, the system's internal energy changes in accord with the law of conservation of energy. The second law of thermodynamics also negates the possibility of perpetual motion; it states that in a natural thermodynamic process, the sum of the entropies of the interacting systems increases. Lastly, the third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature nears absolute zero.
Example Question #3 : Thermodynamics
Calculating heat
How much heat is absorbed by a copper penny as it warms from 




Use the equation that relates heat, mass, specific heat, and change in temperature:
Example Question #3 : Thermodynamics
How do we know if a reaction is spontaneous?
The value of 
The first law of thermodynamics
The sign of
The sign of
The second law of thermodynamics
The sign of
A reaction is spontaneous if and only if 

Example Question #8 : Thermodynamics
What is the standard change in free energy for a reaction run at 

In this question, we're told of a reaction that is run at a given temperature and with a certain equilibrium constant. We're asked to evaluate the standard change in free energy for this reaction.
First, right off the bat, we can see that the equilibrium constant for the reaction is greater than one. As a result, we know that under standard conditions, equilibrium of this reaction will favor products. Hence, we know right away that 
To actually calculate the value of 
If we plug in the values that we know, we can solve for the correct answer.
Example Question #9 : Thermodynamics
Calculate the change in free energy, in kilojoules, of the reaction at 


Recall how to find the change in free energy for a reaction from using free energies of formation:

Where 
Plug in the given values to find the change in free energy for the reaction.
Example Question #1 : Gibb's Free Energy
Suppose that a given chemical reaction has an enthalpy change of 

For this question, we're asked to determine the temperature needed for a reaction to be in a state of equilibrium. We are also provided with the entropy and enthalpy changes for this reaction.
Remember that for a reaction to be in a state of equilibrium, the value for its change in free energy must be equal to zero. Furthermore, we can state the relationship between the change in free energy with the change in entropy, the change in enthalpy, and the temperature as follows.
Because the 
Now that we have this expression, we just need to plug in the two values given to us in the question stem. Remember to make the units match though!
All College Chemistry Resources