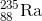All College Chemistry Resources
Example Questions
Example Question #261 : College Chemistry
If 

For this question, we're told that 

The first thing we need to do is write an expression for radioactive decay. Remembering that radioactive decay processes follow a first-order reaction rate, we can use the following expression.
We can further rewrite this expression as follows.
Also, recall that in the beginning we are starting with 




Now that we have the rate constant for the decay reaction, we're equipped with what we need in order to calculate the half-life.
To solve for the half-life, we can derive an expression using one already shown above.
Now, using the above expression, we can plug in the value for the rate constant that we calculated in order to solve for the half-life.
Example Question #261 : College Chemistry
What is the daughter nuclide when 
Recall that beta decay occurs when the nucleus of the parent atom emits an electron. We can then write the following equation to illustrate the beta decay of thorium.
Make sure that the atomic numbers and masses add up to the same on both sides of the equation.
Example Question #263 : College Chemistry


In order to solve this problem we first must setup an equation representing the decay of 


where 

and 
Let's start with the atomic number:
Since we have an atomic number of 



As an equation this is written as:
This simplifies to 


Now we must solve for the mass number similarly:
Following the instructions above except for the mass number we create an equation with one unknown to solve for the mass number of 
therefore
This means our final answer is 
This can be double-checked by plugging the mass number and atomic number into the decay equation and simplifying it.
Example Question #264 : College Chemistry
Which of the following sequences describe the decay process from 

Beta, gamma, alpha, alpha
Alpha, beta
Beta, beta
Alpha, alpha, beta
Beta, beta, beta
Beta, beta, beta
In order to determine the decay process that occurs we should check if the mass number charges or not in the decay. Since the mass number doesn't change we can eliminate any answer with 
Now we can write out our decay equation. Since the atomic numbers aren't written in the question we must find them on the periodic table, where elements are ordered by their atomic number. In the case of Thallium, we find that it is element number 

So our equation is as follows:
where 

and 
Since the mass number is the same on both sides of the equation we know that it is equal to 
Now we see that the atomic number increases by 






Example Question #265 : College Chemistry

Positron emission
Alpha (
Electron capture
Beta (
Gamma emission (
Alpha (
Let's first look at the cases and determine which ones can maintain both the same atomic and mass number. We know that 


As for 

In electron capture, an electron is gained so the atomic number decreases, so this cannot be the right answer either.
Positron emission means a positron 
Therefore the right answer is gamma 
Example Question #1 : Radioactive Decay
When a 


In order to begin determination of the mass number of phosphorus, we must first write out the equation and then solve for the mass number of phosphorus.
We know there is 












Now let's write out the reaction that occurs and all of its reactants and products, so we can solve for X:

Now we can solve for the unknown mass number of phosphorus by adding up the mass numbers on each side of the equation and solving for the unknown.
On the reactants side we have 



This leaves us:
Therefore the mass number of phosphorus is 
Example Question #2 : Radioactive Decay

In an electron capture, an electron is absorbed meaning the atomic number of the product is reduced by 1. Therefore we can write out our radioactive decay equation as:



Where 

and 
Since the mass number doesn't change at all 





Example Question #268 : College Chemistry


In 



Where 

and 
We can quickly determine the atomic number/element name by making an equation with an unknown with all the atomic numbers. This would be:
therefore the atomic number is 
We can do the same for mass numbers to get the equation:


Example Question #2 : Radioactive Decay
What is the daughter nuclide after 
Recall what an alpha particle is: 
Now, write the equation for the alpha decay of polonium-214. The polonium will be emitting an alpha particle.
Make sure that the masses and the atomic numbers on both sides of the equation will add up.
Example Question #261 : College Chemistry
This is a question regarding radioactive decay, specifically alpha decay. An alpha particle has the same identity as the nucleus of a 








All College Chemistry Resources