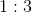All AP Physics 1 Resources
Example Questions
Example Question #1231 : Ap Physics 1
If 


None of these answers
None of these answers
First lets set up two axes. Have 

Coloumb's law tells us the force between point charges is
The net force on charge 2 can be determined by combining the force on charge 2 due to charge 1 and the force on charge 2 due to charge 3.
Since charge 1 and charge 2 are of opposite polarities, they have an attractive force; therefore, charge 2 experiences a force towards charge 1 (in the 


Since charge 2 and 3 have the same polarities, they have a repulsive force; therefore, charge 2 experiences a force away from charge 2 (in the 


If we draw out these two forces tip to tail, we can construct the net force:

From this, we can see that 

Example Question #1231 : Ap Physics 1
Two electric charges are placed 


What is the magnitude of force between them? Is it replusive or attractive?
The force between the two charged particles is proportional to the product of their charges, according to Coulomb's Law. Whether the force is attractive or repulsive depends on the signs of the charges. Like signs will repel while opposite signs will attract.
Using Coulomb's Law to find the magnitude of the charge:
Therefore, the magnitude of the force has been discovered. Finally, since the signs are opposite (

Example Question #1232 : Ap Physics 1
Which of the following pairs of charges would exhibit the most electrostatic repulsive force?


















The correct answer is the 


This results in the largest repulsive force according to the following equation:







Example Question #11 : Electricity
Two protons are on either side of an electron as shown below:

The electron is 30 µm away from the proton on its left and 10 µm away from the proton on its right. What is the magnitude and direction of the net electric force acting on the electron?
A proton has a charge of






The net force on the electron is the sum of the forces between the electron and each of the protons:
These forces are given by Coulomb's law:
Using the numbers given, we get:
Because opposite charges attract, 

Therefore, the net force is
Because this value is positive, the direction is rightward.
Example Question #1 : Ap Physics 2
Charges A and B are placed a distance of 




What is the ratio of 

This question is very simple if you realize that the force experienced by both charges is equal.
The definition of the two electrostatic forces are given by Coulomb's law:
In this question, we can rewrite this equation in terms of our given system.
It doesn’t matter if the charges of the two particles are different; both particles experience the same force because the charges of both particles are accounted for in the electrostatic force equation (Coulomb's law). This conclusion can also be made by considering Newton's third law: the force of the first particle on the second will be equal and opposite the force of the second particle on the first.
Since the forces are equal, their ratio will be 
Example Question #1231 : Ap Physics 1
An excess charge of 



Two principal realizations help with solving this problem, both derived from Gauss’ law for electricity:
1) The excess charge on an ideal conducting sphere is uniformly distributed over its surface
2) A uniform shell of charge acts, in terms of electric force, as if all the charge were contained in a point charge at the sphere’s center
With these realizations, an application of Coulomb’s law answers the question. If 

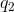
In this equation, 



Using the given values in this equation, we can calculate the generated force:
Example Question #3 : Coulomb's Law
If the distance between two charged particles is doubled, the strength of the electric force between them will __________.
be quartered
quadruple
double
remain unchanged
be halved
be quartered
Coulomb's law gives the relationship between the force of an electric field and the distance between two charges:
The strength of the force will be inversely proportional to the square of the distance between the charges.
When the distance between the charges is doubled, the total force will be divided by four (quartered).
Example Question #1 : Coulomb's Law
If we have 2 charges, 








Use Coulomb's Law
Plug in known values and solve.
A negative value for electric force indicates an attractive force. This makes sense since our two charges have opposite signs. Since we're asked for magnitude, all answer choices are positive.
Example Question #81 : Electricity And Waves
If we have 2 charges, 








Use Coulomb's law.
Plug in known values and solve.
Note that this force is positive, which means it's repulsive.
Example Question #3 : Coulomb's Law
If we have 2 charges, 








Use Coulomb's law.
Plug in known values and solve.
Note that this force is positive, which means that it's repulsive.
All AP Physics 1 Resources