All AP Chemistry Resources
Example Questions
Example Question #3 : Cell Potential Under Non Standard Conditions
Calculate the standard cell potential of the following reaction:
3 F2 (g) + 2 Au (s) -> 6 F- (aq) + 2 Au3+
Given:
F2 (g) + 2 e- -> 2 F- (aq) Eo = 2.87 V
Au3+(aq)+ 3 e--> Au (s) Eo = 1.50 V
-1.37 V
4.37 V
-5.61 V
1.37 V
5.61 V
1.37 V
Eocell = Eo cathode - Eoanode
Eocell = 2.87 – (1.50) = 1.37 V
Example Question #6 : Cell Potential Under Non Standard Conditions
Determine the Ecell for the following reaction at 25 C:
Zn (s) + 2 VO2+ (aq) + 4 H+ -> 2 VO2+ (aq) + Zn2+(aq) + 2 H2O (l)
Given that:
VO2+ (aq) + 2 H+ (aq) + e- -> VO2+(aq) + H2O (l) Eo = 1.00 V
Zn2+ (aq) + 2 e--> Zn (s) Eo = -0.76 V
And
[ VO2+] = 2.0 M; [H+] = 0.50 M; [VO2+] = 1.0 x 10-2M; [Zn2+] = 1.0 x 10-1M
3.71 V
0.95 V
1.76 V
1.89 V
2.41 V
1.89 V
Example Question #1 : Electrolysis And Faraday's Law
How many grams of Cr can be obtained by the electrolysis of a Cr(NO3)3 if 10 amps are passed through the cell for 6 hours?
19.4 g
56.3 g
103 g
38.8 g
12.5 g
38.8 g
Example Question #1 : Electrolysis And Faraday's Law
How much Al would be precipitated if an AlCl3 solution was electrolyzed for 1.00 hours with 5.00 amps?
6.72 g
1.68 g
1.25 g
3.36 g
0.84 g
1.68 g
Example Question #131 : Thermochemistry And Kinetics
What current must be past through a solution of AlCl3 for 1.19 hours to produce 40g of solid Al?
125 amps
50.0 amps
10.5 amps
75.3 amps
100. amps
100. amps
Example Question #3 : Electrolysis And Faraday's Law
In the electrolysis of CaCl2 a 1.15 amp current is passed through the cell for 5.0 hours. How much Ca is produced?
4.3 g
8.6 g
2.2 g
1.1 g
18.3 g
4.3 g
Example Question #4 : Electrolysis And Faraday's Law
How long would it take to electroplate 28.3g of silver at a constant current of 2.0 amps from a solution of silver nitrate?
105 min
844 min
53 min
211 min
422 min
211 min
Example Question #951 : Ap Chemistry
The following diagram shows how the energy changes as the chemical reaction


A chemical engineer wants to, both, increase yield and accelerate this reaction rate. He should __________.
Use a catalyst only
extract 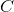
increase the temperature only
extract 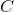
extract 
"Increase the temperature" is incorrect because we have an exothermic reaction and by increasing the temperature the equilibrium constant will become smaller. An increase in temperature will shift the equilibrium toward reagents. According to the Le Chatelier's principle, by extracting 

Example Question #1 : Reaction Energy Profile
If the activation energy of the forward reaction is greater than the activatoin energy of the reverse reaction, then this reaction is __________.
endothermic
exothermic
spontaneous
uncatalyzed
endothermic
If the activation energy of a forward reaction is greater than that of the reverse reaction, thenthe products must have a higher enthalpy than the reactants (draw a potential energy diagram to visualize).
Example Question #2 : Reaction Energy Profile
If the activation energy of a forward reaction is greater than the activation energy of a reverse reaction, what must be true of the reaction?
It is nonspontaneous
It is exothermic
It is spontaneous
It is at equilibrium
It is endothermic
It is endothermic
If the activation energy of the forward reaction is greater than that of the reverse reaction, the products must have a higher enthalpy than the reactants. The net enthalpy change is therefore positive, meaning that it is endothermic.
All AP Chemistry Resources



![E_{cell} = 1.76 V - \frac{ 0.0592}{2} \: log \: \frac{[VO^{2+} ]^2 [Zn^{2+} ]}{[VO_2^+ ]^2 [H^+ ]^4 )}](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172347/gif.latex)
![= 1.76 V - \frac{0.0592}{2}\: log \: \frac{[1.0 x 10^{-2} ]^2 [1.0 x 10^{-1} ]}{[2.0]^2 [0.5]^4} = 1.89](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172348/gif.latex)

















