All AP Chemistry Resources
Example Questions
Example Question #951 : Ap Chemistry
The following diagram shows how the energy changes as the chemical reaction


A chemical engineer wants to, both, increase yield and accelerate this reaction rate. He should __________.
Use a catalyst only
extract 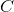
increase the temperature only
extract 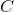
extract 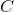
"Increase the temperature" is incorrect because we have an exothermic reaction and by increasing the temperature the equilibrium constant will become smaller. An increase in temperature will shift the equilibrium toward reagents. According to the Le Chatelier's principle, by extracting 

Example Question #1 : Reaction Energy Profile
If the activation energy of the forward reaction is greater than the activatoin energy of the reverse reaction, then this reaction is __________.
endothermic
exothermic
spontaneous
uncatalyzed
endothermic
If the activation energy of a forward reaction is greater than that of the reverse reaction, thenthe products must have a higher enthalpy than the reactants (draw a potential energy diagram to visualize).
Example Question #2 : Reaction Energy Profile
If the activation energy of a forward reaction is greater than the activation energy of a reverse reaction, what must be true of the reaction?
It is nonspontaneous
It is exothermic
It is spontaneous
It is at equilibrium
It is endothermic
It is endothermic
If the activation energy of the forward reaction is greater than that of the reverse reaction, the products must have a higher enthalpy than the reactants. The net enthalpy change is therefore positive, meaning that it is endothermic.
Example Question #3 : Reaction Energy Profile
Which of the following is true regarding activation energy?
I. Activation energy depends on the pressure of the system
II. The reaction rate decreases as the activation energy increases
III. Catalysts increase the activation energy of a reaction
II only
I only
I and II
I and III
II only
Activation energy is the energy barrier that needs to be overcome for a reaction to proceed; the higher the activation energy, the slower the reaction. Activation energy can only be altered via a catalyst. Catalysts are chemical substances that lower the activation energy, allowing reactions to proceed faster. Other physical quantities such as temperature and pressure don’t alter the activation energy.
Reaction rate is a direct measure of the speed of a reaction. As mentioned, increasing activation energy will increase the barrier and, therefore, slow down the reaction.
Catalysts are chemical substances that decrease the activation energy, thereby increasing the reaction rate. They are commonly used in chemical reactions to drastically speed up reactions that might otherwise take hours or days to complete. Enzymes are biological catalysts that facilitate most of the biological reactions happening in our bodies.
Example Question #4 : Reaction Energy Profile
__________ the activation energy of a reaction will __________ the amount of products produced.
Decreasing . . . increase
Increasing . . . increase
Decreasing . . . decrease
Increasing . . . not change
Increasing . . . not change
This question is asking about the relationship between activation energy of a reaction (kinetics) and the amount of products produced (equilibrium). Remember that altering the speed of a reaction (kinetics) does not change the equilibrium of the reaction. Increasing or decreasing activation energy (which alters the speed of reaction) will simply allow for the reaction to proceed slower or faster, respectively. It will not change the amount of products produced at the end.
Example Question #3 : Reaction Energy Profile
Which of the following is the correct way to calculate the activation energy of a reaction?
Energy of reactant – Energy of transition state = Activation energy
Energy of product – Energy of transition state = Activation energy
Energy of transition state – Energy of reactant = Activation energy
Energy of transition state – Energy of product = Activation energy
Energy of transition state – Energy of reactant = Activation energy
Activation energy is the difference between the energy of the transition state and the energy of the reactant. Recall that activation energy is the energy barrier that needs to be overcome by a reaction. The transition state is a higher-energy, intermediary molecule that lies in between the reactants and products. It is created when reactants are in the process of becoming products. Therefore, to get to products, it is necessary to go through this high-energy transition state.
Activation energy is the energy needed to climb this energy “hill” (energy needed to go from reactants to transition state); therefore, the activation energy is the energy of the transition state (top of the energy hill/barrier) minus the energy of the reactant.
Example Question #1 : Reaction Energy Profile
Consider the following reaction:
The conversion of 



What is the activation energy of the reverse reaction?
Cannot be determined from the given information
To answer this question, we have to consider an energy diagram for an exothermic reaction. We know the forward reaction is exothermic because the question states that energy is released. We are given the activation energy of the reaction (the energy difference between the activated complex, or transition state, and the reactants) and the energy released (the energy difference between the products and the reactants). In the reverse reaction we will be going from products to reactants as follows:
This means that the activation energy for reverse reaction will be higher (has to climb a higher hill from “products” to “activated complex”). Using the given information we can deduce that the activation energy (
The closest answer is 


Example Question #51 : Kinetics And Energy
Which of the following is true regarding activation energy?
Activation energy determines the equilibrium constant of a reaction
A catalyst increases the energy of the reactants
Enthalpy of a reaction has no effect on the activation energy
Increasing the temperature will decrease the activation energy
Enthalpy of a reaction has no effect on the activation energy
Recall that the only thing that can alter the activation energy of a reaction is the addition of a catalyst. Factors such as enthalpy, entropy, temperature, and pressure don’t change the activation energy.
Catalysts decrease the activation energy and increase the rate of reaction. They have no effect on the energy of the reactants. As mentioned, temperature will not alter the activation energy; however, increasing the temperature will speed up the reaction. This occurs because adding energy in the form of heat will increase the energy of individual molecules in the reaction and will allow more molecules to overcome the energy barrier. Note, however, that temperature does not change the energy barrier (activation energy). Recall that changing activation energy has no effect on the equilibrium of the reaction; therefore, equilibrium constant does not depend on the activation energy.
Certified Tutor
Certified Tutor
All AP Chemistry Resources










