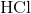All SAT II Chemistry Resources
Example Questions
Example Question #1 : Compounds
Which of the following are strong electrolytes?
Possible Answers:
Three
Two
Four
All five
One
Correct answer:
Three
Explanation:
Strong electrolytes are substances that exists in solutions as only ions. These substances dissociate 100% in solutions. Strong acids, strong bases, and salts are all examples of strong electrolytes. 




Taryn
Certified Tutor
Certified Tutor
University of Florida, Bachelor of Science, Biology, General. University of Florida, Bachelor of Science, Business Administra...
Geo
Certified Tutor
Certified Tutor
Church Teachers College, Bachelor, Science Education. University College of the Carib, Bachelor in Business Administration, B...
All SAT II Chemistry Resources
Popular Subjects
Math Tutors in San Francisco-Bay Area, Spanish Tutors in Houston, Physics Tutors in Seattle, MCAT Tutors in Chicago, Math Tutors in Denver, Calculus Tutors in San Diego, SAT Tutors in New York City, Statistics Tutors in San Francisco-Bay Area, Spanish Tutors in Boston, ACT Tutors in San Diego
Popular Courses & Classes
ACT Courses & Classes in San Diego, MCAT Courses & Classes in Atlanta, GRE Courses & Classes in Boston, MCAT Courses & Classes in Seattle, LSAT Courses & Classes in Boston, ISEE Courses & Classes in New York City, ISEE Courses & Classes in Atlanta, GRE Courses & Classes in Phoenix, GRE Courses & Classes in Houston, SAT Courses & Classes in New York City
Popular Test Prep
MCAT Test Prep in Washington DC, MCAT Test Prep in Denver, SAT Test Prep in Los Angeles, GRE Test Prep in Washington DC, GRE Test Prep in Los Angeles, LSAT Test Prep in Dallas Fort Worth, ISEE Test Prep in Phoenix, SAT Test Prep in Chicago, GRE Test Prep in New York City, GMAT Test Prep in Miami