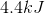All Physical Chemistry Resources
Example Questions
Example Question #21 : Physical Chemistry
What amount of heat must be added to a 20-gram sample of ice in order to raise the temperature from 

Possible Answers:
Correct answer:
Explanation:
This question requires three steps:
1. We need to raise the temperature of the ice from 

2. We need to melt the ice.
3. We need to raise the temperature of the water from 

Steps one and three can use the equation:
Step 2 requires the enthalpy of fusion to be multiplied by the mass of the water sample.
Finding these three added heats will give us the total amount of heat needed to raise the temperature from 

Mehak
Certified Tutor
Certified Tutor
Guru Nanak Dev University, Bachelor of Science, Chemistry. Guru Nanak Dev University, Master of Science, Chemistry.
All Physical Chemistry Resources
Physical Chemistry Tutors in Top Cities:
Atlanta Physical Chemistry Tutors, Austin Physical Chemistry Tutors, Boston Physical Chemistry Tutors, Chicago Physical Chemistry Tutors, Dallas Fort Worth Physical Chemistry Tutors, Denver Physical Chemistry Tutors, Houston Physical Chemistry Tutors, Kansas City Physical Chemistry Tutors, Los Angeles Physical Chemistry Tutors, Miami Physical Chemistry Tutors, New York City Physical Chemistry Tutors, Philadelphia Physical Chemistry Tutors, Phoenix Physical Chemistry Tutors, San Diego Physical Chemistry Tutors, San Francisco-Bay Area Physical Chemistry Tutors, Seattle Physical Chemistry Tutors, St. Louis Physical Chemistry Tutors, Tucson Physical Chemistry Tutors, Washington DC Physical Chemistry Tutors
Popular Courses & Classes
LSAT Courses & Classes in San Francisco-Bay Area, ISEE Courses & Classes in Denver, GMAT Courses & Classes in Miami, GRE Courses & Classes in Seattle, ISEE Courses & Classes in Phoenix, Spanish Courses & Classes in Atlanta, LSAT Courses & Classes in Seattle, SSAT Courses & Classes in Los Angeles, MCAT Courses & Classes in Los Angeles, GRE Courses & Classes in San Diego
Popular Test Prep
ISEE Test Prep in Philadelphia, MCAT Test Prep in Phoenix, SAT Test Prep in Atlanta, MCAT Test Prep in San Francisco-Bay Area, GMAT Test Prep in Atlanta, SAT Test Prep in Washington DC, GRE Test Prep in Washington DC, SSAT Test Prep in San Francisco-Bay Area, GRE Test Prep in Seattle, LSAT Test Prep in Seattle