The Varsity Tutors Organic Chemistry Mobile App
Organic chemistry is considered to be one of the most difficult courses you can take as an undergrad. However, if you’re going into the health and science fields -- particularly if you are an aspiring medical student -- you must take and conquer this class. You will be introduced to such topics as organic functional groups, naming organic compounds, organic structure, and multi-step organic synthesis, among many others.
The free Varsity Tutors Organic Chemistry app for iPhone, iPad, and Android devices can help you dig into the concepts you’ll need to know, whether you simply need to review or want to learn an entirely new topic. The app helps you study anywhere, anytime! With handy tools like flashcards and practice tests, the app helps you to fit your studies into any amount of time you can spare as you go through your busy day.
The first thing you’ll learn in organic chemistry is the mastery of laboratory practices, such as learning how to identify, purify, and quantify compounds. Then, you will look at organic concepts, including biological molecules, intermolecular forces and stability, nucleophiles and electrophiles, organic functional groups and molecules, and reaction types.
Next, you will delve into reactions, including elimination, substitution, and other reaction mechanisms like electron pushing. Then, you’ll look at reactions by amino, carbonyl, and hydrocarbon products, and reactions by amino, carbonyl, and hydrocarbon reactants. You will also learn to work with reactions based on their specific names, such as aldol condensation, keto-enol tautomerization, esterification, and haloform and Wittig reactions.
When you reach the redox chemistry section, you will test your knowledge on organic oxidizing agents like dichromatic compounds; organic reducing agents including lithium aluminum hydride, and oxidation-reduction reactions. The course also covers stereochemistry, which includes benzene additions and isomers, like cis-trans isomers, conformers, diastereomers, enantiomers, epimers, and meso compounds.
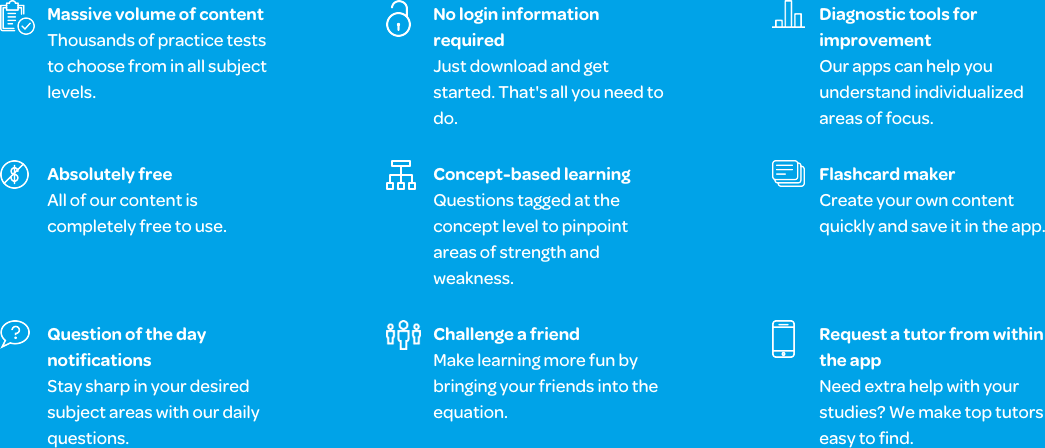
The free Varsity Tutors Organic Chemistry app, available for both iOS and Android devices, offers many tools that may help you increase your knowledge of organic chemistry. First, you can take one of the full-length, timed practice tests, which will give you an in-depth look at where your strengths and weaknesses are. You can also use the test to learn concepts. For each incorrect answer, a comprehensive explanation is given for the right answer, fostering a learning of new material as you go. There are a wide variety of these tests available, so you can take them throughout the course and save your scores to keep track of your progress.
Once you’ve learned where you should focus your studies, try the Learn by Concept resource, which includes questions and answers broken down into very specific categories. You can also utilize the app’s flashcards, or, if you prefer, you can make your own. The Flashcard Maker helps you create custom cards with your own images, audio, and text for a more personalized learning experience.
Download the free Varsity Tutors Organic Chemistry app from iTunes or the Google Play Store today for constant, convenient access to interactive study materials.
66 mobile apps to choose from for your tutoring needs.
Learn More
Organic Chemistry, while difficult, is a vital course if you are preparing for the science and health fields. This area of study focuses on how carbon-containing compounds are formed, used, and interacted with by other materials. Though it previously focused solely on living organisms, it has expanded to include human-made substances. There are many fields organic chemistry can be applied in, such as biotechnology, chemical, manufacturing, pharmaceutical, petroleum, agriculture, personal care, and others. You must have taken AP Chemistry and earned the college-level credit to progress into organic chemistry in your freshman year.
At the end of the two-semester class, you will have taken two final exams. During the first exam, you will need to be capable of providing a specific example of various observations, types, and measurements conducted in the early ages of organic chemistry. These must be backed up with facts, figures, and experiments. Further, you will need to analyze structures on a molecular level, comprehend quantum points of view, demonstrate the ability to explain complex formulas, discuss the Cahn-Ingold-Prelog system, and identify important historical figures in the subject. The second exam will focus on assessing your understanding of the topics covered during the second semester, such as alcoholic compounds, alkene reactions, the structure and reaction of aromatic compounds, and more.
To start the course, you will learn about standard laboratory practices used in organic chemistry and what they can tell you about compounds. Your professor will show you how to identify, purify, and quantify compounds using a variety of techniques and strategies. You will learn how to interpret lab data and what methods to employ to analyze a compound based on observed characteristics and reactions.
Digging into your first organic concepts, your instructor will introduce you to biological molecules. Biological molecules can be found in all living organisms. You will learn to be able to identify and work with organic proteins, lipids, and carbohydrates. Other biomolecules, such as amino acids, will also be covered. In addition, you will learn to identify organic reactions, understand intermolecular forces, and be capable of noting the differences between electron-donating and electron-withdrawing.
From there, your instructor will delve into organic molecules and functional groups. You will learn to identify the properties of different organic compounds and know the characteristics of the different groups of organic intermediates. It is important that you are capable of understanding these compounds on a deeper level, as they will tie into the following concepts as you go further.
As you progress in the course, you will be introduced to the many properties of molecules. There are several principles that are important in this part of the class. You'll discuss the important acid and base formulas and the inductive effects related to acidity, as well as trends related to hybridization, resonance, and attached atoms. In addition, your instructor will focus some time on Bronsted-Lowry Acidity, Lewis Acidity, and bond strengths and lengths.
You may spend a few days on the concept of alkanes, cycloalkanes, and stereochemistry. You will work with molecular formulas for constitutional isomers and degrees of unsaturation. You'll dig into butane, ethane, and propane fuel; perform conformational analysis on each; and learn about Newman Projections. There are many principles related to cycloalkanes that are important to the course, such as ring sizes and strains. You will learn about analyzing a variety of complicated compounds, including cyclobutane. In addition, you'll learn about mono-substituted cyclohexane and the axial versus equatorial groups. You'll focus on the basics associated with bicyclic ringing systems and di-substituted cyclohexanes, such as cis/trans isomerism and preferred conformers.
In the final section of your first semester of organic chemistry, you will learn about free radical reactions within a variety of different concepts. You will come to know the mechanism associated with chlorinating methane, as well as go back over thermodynamics and kinetics. In addition, your professor will discuss Hammond’s postulate and multi-step reactions. Lastly, you'll learn about chlorination and bromination, including selectivity and relative reactivity.
The second semester of organic chemistry appears to cover fewer concepts, though it is no less rigorous than the previous semester. To start with, you'll learn about substitution and elimination mechanisms and reactions. First, you'll dive into the nomenclature, structure, and basic substitution and elimination options. From there, your professor will focus on reactions based on the product. There are amino, carbonyl, and hydrocarbon products. Amino uses amine synthesis. However, you will need to be able to adeptly discuss carbonyl products with alcohol, aldehyde, carboxylic acid, and other synthesis materials. During this section, you may be required to analyze the various products, test a hypotheses based on the reactions, and perform other experimentation.
Next, you will focus on reactions based on the reactant. You will learn to identify and work with amino reactions, both amide and nitro. You will be instructed in how to detail the structure and bonding of alcohol molecules, as well as how chemists are able to synthesize different types of alcohols through old and new strategies. You'll learn about organometallic reagents, how they react to one another, and their acidity and basicity. You will spend time on how the compounds react to Grignard agents, as well as organolithium. Some of the most important concepts of this section of organic chemistry are the REDOX relationships between ketones, aldehydes, alcohols, and carboxylic acids.
Following the above, your professor will spend some time specifically on redox chemistry. Redox chemistry, or oxidation-reduction, discusses an important aspect of oxidation and chemical reactions. You will learn to identify similarities between reactions, as well as their differences. In addition, your professor will teach you about the various organic oxidizing agents, the oxidizing reducing agents, and the reactions between the two.
The next major section of organic chemistry covers the specific reactions. You will be taught about each of the different potential reactions that are possible, such as hydrocarbon and carbonyl, and the concept of reduction reactions. You will also need to learn the definitions, explanations, and applications of Wolff-Kishner Reduction and Grignard Reaction.
The last few weeks of organic chemistry are spent focused on stereochemistry. There are many types of benzene additions that you will learn about identifying, such as meta, ortho, and para. In addition, you will hone your prediction capabilities based on data. Isomers are another big principle related to stereochemistry. There are six types of isomers that you will need to be capable of recognizing on the final exam.
Organic chemistry is one of the most difficult courses you will take as an undergraduate student. However, it is a valuable course that can be used to gain admittance into science and health programs around the country.




