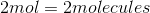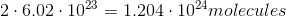All High School Chemistry Resources
Example Questions
Example Question #1 : Units
Consider the following four samples:




Which of the given samples contains the most atoms?
Lithium
They all have the same number of atoms
Chlorine
Potassium
Lithium
It is important to note that the mass of a sample does not tell you the amount of atoms in the sample. The number of atoms in a sample is dependent on the number moles in a sample, given by Avogadro's number. Here is the number of moles for each sample:
Remember that chlorine is a diatomic mass, so each molecules contains two atoms. This doubles the molar mass for the conversion.
The sample with the greatest number of moles will also contain the most atoms. In this case, the sample of lithium results in the largest number of moles and, thus, the greatest number of atoms.
Example Question #4 : High School Chemistry
Consider the reaction above. If you start with 
In the chemical equation, the ratio of potassium bromide to bromine is 2:1, so for every 2 moles of 
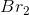


Example Question #5 : High School Chemistry
Convert the following amount from grams (g) to moles (m)
How many moles is 

Use the periodic table to calculate the molecular weight of sodium hydroxide.
Next, use dimensional analysis to find the number of moles.
Example Question #6 : High School Chemistry
How many moles of 

The molecular weight of carbon dioxide is 

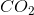

All High School Chemistry Resources