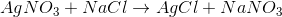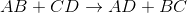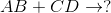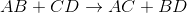All High School Chemistry Resources
Example Questions
Example Question #2 : Types Of Reactions
What is the type of reaction seen below?
Addition
Decomposition
Double-replacement
Single-replacement
Double-replacement
By looking at the reaction, we see that the cations for the reactants have their anions switched in the products. This means that the reaction follows the general outline:
This is an example of a double-replacement reaction.
Addition reactions convert two reactants to a single product, while decomposition reactions convert a single reactant to multiple products. Single-replacement reactions only switch one cation-anion pair.
Example Question #2 : Help With Double Replacement Reactions
Predict the products for the following reaction if it were double displacement.
In a double displacement reaction, or double replacement reaction, opposite ions combine. What this means is that the cation of one compound will recombine and bond to the anion of the other compound and vice versa.
In this example, we have to remember the ideal convention of cations being written before the anion in the compound. So in AB, we can presume A being the cation and B being the anion. The same goes for CD - C is the cation and D is the anion.
With this in mind, we can now easily see how the replacement would be possible.
If A is a cation, and was originally bound to B (anion), the only other anion it is left with to bind is D. If C is a cation, and was originally bound to D (anion), it is only left with the option of binding to the now-free B anion.
That's why it is:
All High School Chemistry Resources