All GRE Subject Test: Chemistry Resources
Example Questions
Example Question #1 : Molecular And Electronic Geometries
Which of the following is not the correct geometric configuration for the given molecule?



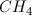


Recall the following relationships between geometry and number of pairs of electrons on the central atom.
2: linear
3: trigonal planar
4: tetrahedral
5: trigonal bipyriamidal
6: octahedral
To visualize the geometry, we need to think of how many electron pairs are on the central atom. Drawing Lewis dot diagrams may be helpful here. None of the answer choices has lone central electron pairs, with the exception of water, so the number of atoms bound to the central atom is the same as the number of central electron pairs.
The only one that does not match up with the correct geometry is SF6, which is actually octahedral since it has six central electron pairs. In a water molecule, the central oxygen has six valence electrons, plus one from each bond with hydrogen, for a total of eight central electrons and four central electron pairs. So, this geometry is a variation on the tetrahedral form (bent), in which two central electron pairs are not bound.
Example Question #53 : General Chemistry
Which of the following molecules exhibits a trigonal pyramidal geometry?
The trigonal pyramidal geometry is implemented by molecules in which the central atom has three atoms and a lone pair attached. 



Example Question #3 : Diagrams And Geometry
Which of the following molecules has an electronic geometry that is the same as its molecular geometry?
Electronic and molecular geometries are only the same when there are no lone pairs around the central atom in the molecule. 
Example Question #11 : Vsepr And Bond Hybridization
What is the molecular shape of 
Trigonal bipyramidal
Octahedral
Trigonal planar
Tetrahedral
Trigonal pyramidal
Trigonal pyramidal
NH3 is trigonal pyramidal because it has 4 electron domains, one of them being a lone pair of electrons and the other three being H atoms. When this is arranged in a three-dimensional space, it is trigonal pyramidal in shape.
Certified Tutor
All GRE Subject Test: Chemistry Resources









