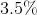All College Chemistry Resources
Example Questions
Example Question #1 : Acid Base Reactions
The titration of a 




Start by writing the chemical equation for this acid-base reaction.
Next, find the number of moles of 
At the equivalence point of a titration, the moles of acid and the moles of base are the same.
Finally, find the molarity of the solution.
Make sure to round the answer to three significant figures.
Example Question #1 : Acid Base Reactions
A 



Start by finding the initial number of moles of sodium hydroxide by using the given volume and molarity. Since sodium hydroxide is a strong base, it will dissociate completely into 
Next, find the number of moles of the hydrochloric acid added by using the given volume and molarity. Since hydrochloric acid is a strong acid, all of the 
Now, as the acid is added, it will neutralize some of the base as shown by the following equation:
Since the reactants are found in a 
Now, divide this number of moles by the total volume of the solution to find the concentration of the base.
Next, find the 
Finally, find the 
Your answer should have 
Example Question #61 : Reactions
![]()
Determine the volume in mL of 





Determine the moles of 
At the equivalence point of the titration:
Convert the moles of 
Convert the liters to milliliters:
Example Question #1 : Titrations
![]()
Determine the volume in mL of 





Determine the moles of 
At the equivalence point of the titration:
Convert the moles of 
Convert the liters to milliliters:
Example Question #1 : Titrations
![]()








Using the concentration of 

At the equivalence point of the titration:
Example Question #2 : Titrations
![]()
Household vinegar contains the organic compound acetic acid with chemical formula, 







At the equivalence point of the titration:
Convert the moles of 
Using the density of 
Determine the percentage of 

Example Question #11 : Acid Base Reactions
![]()








Using the concentration of 

At the equivalence point of the titration:
Example Question #1 : Titrations
![]()
Household vinegar contains the organic compound acetic acid with chemical formula, 







Convert the liters of 
At the equivalence point of the titration:
Convert the moles of 
Using the density of 
Determine the percentage of 

All College Chemistry Resources

















![[\text{OH}]^-=\frac{7.5\times 10^{-4}\text{ moles}}{0.075\text{L}+0.045\text{L}}=0.00625\text{ M}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/902579/gif.latex)