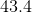All College Chemistry Resources
Example Questions
Example Question #2 : Thermochemistry And Changes Of State
A 



Recall the equation that gives the relationship between change in temperature and amount of heat:

where 


Since the question asks for the final temperature, re-arrange the equation to solve for 
Substitute in the given values to solve for the final temperature.
Example Question #3 : Solutions, States Of Matter, And Thermochemistry
The specific heat capacity is defined as the amount of heat energy necessary to change a given amount of a substance by a certain temperature. Which of the following correctly expresses the units of specific heat capacity?
For this question, we're given a definition for the specific heat capacity of a substance and we're asked to identify the correct units for this term.
We can also recall the equation that relates all of these terms.
Rearranging this expression to isolate the term for the specific heat capacity gives us the following.
Next, we can recall what units would be appropriate to use for each of the following terms in the above expression. The 


Putting all this together gives us the following.
Example Question #31 : College Chemistry
How much heat is needed to raise the temperature of 10.0 g of water from 10.0 oC to 35.0 oC?
Specific heat capacity for water is
Recall the relationship between heat and specific heat capacity
Plug in known values and solve for Q
Example Question #32 : College Chemistry
An insulated container is filled with 50.0 g of water at 15.0 oC. 120.0 g of lead is heated to 100 oC and added to the insulated container. What is the final temperature of the system once it comes to equilibrium?
Specific heat of water is
Specific heat of lead is
Since Pb starts at a higher temperature than the water, we know that energy (in the form of heat) will be transferred from Pb to water. Due to the law of conservation of energy, the exact same amount of energy lost by Pb must be gained by water.
Recall that
Combining the two equations, we have
Combine like terms then solve for final temperature
Example Question #1 : Specific Heat Capacity
How much heat does it require to make a 



Recall the equation that gives the relationship between the change in temperature and the amount of heat:



Substitute in the given values to find how much heat is required to increase the temperature of the block of lead the specified amount.
Make sure to round the answer to three significant figures.
Certified Tutor
All College Chemistry Resources