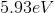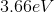All AP Physics 2 Resources
Example Questions
Example Question #1 : Quantum And Nuclear Physics
A physicist is trying to determine the identity of a metal by experimentally finding its work function and then comparing the experimental value to a list of known values. If the physicist shines light with a frequency of 

To determine the work function of the metal, we can begin by calculating the kinetic energy of the electron from its speed.
Then, convert joules into electron volts:
Next, we'll calculate the energy of the light by using its frequency according to the equation:
Then, we can convert this energy into electron volts:
And finally, we can use the equation for work function:
Example Question #2 : Photons And Photon Energy
An atomic transition takes place from 

The energy difference between the second excited state 

Since the energy difference is in electron volts, they need to be converted to Joules. Another convenient formula is to notice that since 

Example Question #6 : Principles Of Quantum Mechanics
It is possible that electrons can be ejected from a metal plate by light. Which of the following properties of light accounts for this phenomena?
Intensity
Frequency
Amplitude
Speed
Electric force
Frequency
The ejection of electrons from a metal is known as the photoelectric effect. In fact, it was a key discovery that led to the acceptance of the particle nature of light, because the wave nature of light could not account for this finding.
The key that allowed scientists to find this out was that the speed of electrons ejected from the metal was dependent on the color of the light. The intensity of light had no effect on the speed at which electrons were ejected.
As a part of developing this theory, scientists referred to individual discrete particles of light as photons. In order to eject electrons from the metal, individual photons needed to have a sufficient amount of energy. The intensity of light (with a greater amplitude of its waves) represents a case in which photons are striking the metal at a greater rate. But, the individual energy of the photons could be too low in such a situation, hence why intensity is not the key property here. Instead, a higher frequency of light allows each individual photon to have greater energy - enough to knock the metal's electrons off. Furthermore, as the frequency of the light increases, the energy of the individual photons increases and the kinetic energy of the ejected electrons increases as well. The expression for the energy of a photon is shown as follows.
All AP Physics 2 Resources