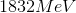All AP Physics 2 Resources
Example Questions
Example Question #7 : Principles Of Quantum Mechanics
How much energy is contained in a particle that has a mass of 
This is an example of one of Einstein's greatest ideas: the relation between the mass of an object/particle, and the energy contained by the mass. This is given as
In order to calculate the energy in our particle, we must make sure that the mass is in units of 
Now we can plug in numbers to our equation and solve for energy.
Example Question #1 : Principles Of Quantum Mechanics
Suppose that the mass of a neutral Uranium atom is measured and found to be 

In this question, we're presented with information concerning the mass of a uranium atom. We're told two values: the mass of a uranium atom as measured, and the predicted mass of a uranium atom. We're then asked to determine the nuclear binding energy for uranium.
In order to solve this question, we have to realize the significance of the discrepancy between the observed and predicted mass of uranium. The predicted mass is calculated by adding up the individual masses of each constituent proton, electron, and neutron. However, the reason why the measured mass is less than the predicted mass is due to energy-mass equivalence. When the constituent protons and neutrons come together to form the nucleus, some of their mass is converted into energy, and it is this energy which holds these constituent nucleons together. Because some of the mass is converted into energy, the observed mass is less than what we would predict.
Now that we understand why there is a discrepancy between observed and predicted mass, we can calculate the nuclear binding energy by using Einstein's famous equation.
This equation states that the nuclear binding energy is equal to the difference between observed and predicted mass, multiplied by the speed of light squared. So to solve for energy, we can plug in the values given to us.
Example Question #9 : Principles Of Quantum Mechanics
Two grams of helium are completely converted into energy and used to power a 
The energy from the two grams of helium can be found using
This energy can then be equated to the man's kinetic energy, which can then be used to find the man's velocity.
Example Question #1 : Principles Of Quantum Mechanics
If the combination of protons and neutrons in an atom's nucleus results in a mass defect of 
In this question, we're given the mass defect of an atom's nucleus and are asked to find the binding energy for this atom.
To begin with, it's important to understand that when protons and neutrons come to be held together within the nucleus of an atom, there is a tremendously powerful force holding them together. This incredibly large force accounts for the mass defect. In other words, the total mass of the nucleus is smaller than the sum of the individual masses of the protons and neutrons that make up that nucleus, and this is due to the strong force.
Einstein's mass-energy equivalence explains the observable mass defect; the mass lost is converted into an enormous amount of energy according to the following equation.
But first, we'll need to convert the mass given to us in the question stem into grams.
Furthermore, because we know the value for the speed of light, we can use this information to solve for the binding energy.
Certified Tutor
Certified Tutor
All AP Physics 2 Resources