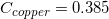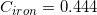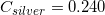All AP Chemistry Resources
Example Questions
Example Question #17 : Reaction Rate And Rate Law


The mechanism for decomposition of ozone is shown. What is the intermediate of the process?
Intermediate is created and destroyed, and therefore does not appear on the net equation, which is 

Example Question #11 : Reaction Rate And Rate Law
The rate law of the reaction, 
![\textup{Rate} = k\left [ A\right ]^{3}](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/770593/gif.latex)
Adding the catalyst to the reaction
Increasing the concentration of
Increasing the temperature of the reaction
Increasing the concentration of
Increasing the concentration of
The reactant 

Example Question #31 : Thermochemistry And Kinetics
Which of the following changes to reaction conditions will always result in an increase in the reaction rate?
Decreased temperature
Decreased concentration of products
Increased concentration of reactants
Increased temperature
Increased temperature
For this question, we're asked to identify something that will always increase the rate of a reaction. Notice that the question specifically states "always."
Let's go through each answer choice and see how it affects the reaction rate.
When the concentration of reactants is increased, this may increase the reaction rate, but not always. For example, if a reaction is zero-order with respect to its reactants, then changing the reactant concentration will have no effect on the rate.
Just like with reactants, decreasing the concentration of products may or may not change the reaction rate. If the reaction rate is zero-order with respect to the products, then a change in their concentration will have no effect on the reaction rate.
A change in temperature is the only thing that is guaranteed to change the reaction rate. This is because changing the temperature will directly change the rate constant of the reaction. Increasing the temperature will increase the rate constant, and hence the reaction rate.
Example Question #1 : Thermodynamics
The following is a list of specific heat capacities for a few metals.
A 50g sample of an unknown metal is heated with 800 joules. If the temperature of the metal increases by 41.6oC, what is the identity of the unknown metal?
Silver
Aluminum
Iron
Copper
Copper
We need to find the specific heat of the unknown sample of metal in order to locate it on the list. We can do this by using the equation that allows us to determine the specific heat capacity of an element.
Since we know the change in temperature, we can simply plug in the values and solve for the value of 
Going back to the list, we see that this is the specific heat capacity for copper, so we confirm that the unknown metal is copper.
Example Question #1 : Thermodynamics
In which instance would a bomb calorimeter be more useful than a coffee-cup calorimeter?
When measuring the specific heat of water vapor
When measuring the mass of a substance
When measuring the heat capacity of a solid
None of the above
When measuring the specific heat of water vapor
Bomb calorimeters are most useful when dealing with a gas, because they can operate well at high pressures. Coffee-cup calorimeters are not useful when water begins to boil, producing vapor.
Example Question #1 : Calorimetry, Specific Heat, And Calculations
How much heat does it take to heat 100g ice at 0C to boiling point?
Cice= 2.1 J/goC
Cwater= 4.2 J/goC
ΔHvap= 2260 J/g
ΔHfus=334 J/g
42 kJ
33.4 kJ
300.5 kJ
75.4 kJ
55.4 kJ
75.4 kJ
You need heat for the phase change, using the enthalpy of fusion (100g*334 J/g = 33400 J). Add to this the heat to get to boiling point using the specific heat of water (100g*100C*4.2 J/goC = 42000 J). Totalling 75400 J (75.4 kJ)
Example Question #4 : Thermodynamics
The specific heat capacity of an unknown liquid is 


None of the available answers
First we will determine the mass of the liquid:
Now we will examine the relationship between heat and specific heat capacity:
Where 



If we begin at 
Example Question #1 : Thermodynamics
A 50g sample of a metal was heated to 




Which of the following can be concluded?
The specific heat of the water is greater than that of the metal
The specific heat of the metal is greater than that of the water
The metal lost an amount of thermal energy that was more than the amount of thermal energy gained by the water
None of the other answers
The water gained an amount of thermal energy that was more than the amount of thermal energy lost by the metal
The specific heat of the water is greater than that of the metal
When the heated metal is placed in the container of the cooler water there will be a transfer of thermal energy from the metal to the water. This transfer will occur towards an equilibrium of thermal energy in the water and in the metal. Thus we can conclude that the amount of thermal energy lost by the metal will equal the amount of thermal energy gained by the water. However we notice that the water increases by only 5oC and the metal decreases by 65oC. This is becasue of the difference of the specific heats of these substances. The specific heat capacity of a substance is the heat required to increase the temperature of 1g of a substance by 1oC. The metal can be conluded to have a smaller specific heat than the water because the same amount of energy transfer led to a much larger change in termperature for the metal as compared to the water.
Example Question #6 : Thermodynamics
Which of the following is the correct molar specific heat of water used when making calculations involving a calorimeter?
418.4 J/gK
4.184 J/gK
41.84 J/gK
0.4184 J/gK
4.184 J/gK
4.184 J/gK is the cited value for the specific heat of water and should be memorized. This is used during calorimeter calculations, specifically when using the equation q= mc delta(T).
Example Question #2 : Thermodynamics
How much energy is needed to raise the temperature of five grams of ice from 

This question involves the total energy needed for three different processes: the temperature raise from 





1.
2.
3.
Finally, we will need to sum the energy required for each step to find the total energy.
Certified Tutor
Certified Tutor
All AP Chemistry Resources