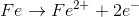All AP Chemistry Resources
Example Questions
Example Question #4 : Oxidation Reduction Reactions
For the redox reaction shown, which of the following half reactions occurs in the anode?
Possible Answers:
Correct answer:
Explanation:
Recall that oxidation always occurs at the anode (in both the electrochemical and galvanic cells). 



Emily
Certified Tutor
Certified Tutor
University of Pennsylvania, Bachelor of Science, Chemical and Biomolecular Engineering.
Yucheng
Certified Tutor
Certified Tutor
The University of Texas at Austin, Bachelor of Science, Mechanical Engineering.
All AP Chemistry Resources
Popular Subjects
Math Tutors in Washington DC, ISEE Tutors in Dallas Fort Worth, GRE Tutors in San Diego, ISEE Tutors in Atlanta, Computer Science Tutors in Atlanta, ISEE Tutors in Philadelphia, Statistics Tutors in Denver, SAT Tutors in Chicago, SAT Tutors in San Francisco-Bay Area, GMAT Tutors in San Diego
Popular Courses & Classes
LSAT Courses & Classes in Denver, LSAT Courses & Classes in Houston, Spanish Courses & Classes in Atlanta, MCAT Courses & Classes in Boston, MCAT Courses & Classes in Houston, ISEE Courses & Classes in Chicago, SSAT Courses & Classes in Seattle, MCAT Courses & Classes in Los Angeles, GRE Courses & Classes in Denver, Spanish Courses & Classes in Philadelphia
Popular Test Prep
SAT Test Prep in San Diego, SAT Test Prep in Los Angeles, LSAT Test Prep in San Diego, SSAT Test Prep in Los Angeles, ISEE Test Prep in Boston, LSAT Test Prep in Phoenix, ISEE Test Prep in Philadelphia, MCAT Test Prep in Dallas Fort Worth, GMAT Test Prep in Washington DC, SAT Test Prep in New York City