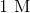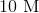All SAT II Chemistry Resources
Example Questions
Example Question #2 : Solutions And States Of Matter
30 mL of 1.0 M 
Possible Answers:
Correct answer:
Explanation:
Recall that 

Taryn
Certified Tutor
Certified Tutor
University of Florida, Bachelor of Science, Biology, General. University of Florida, Bachelor of Science, Business Administra...
Geo
Certified Tutor
Certified Tutor
Church Teachers College, Bachelor, Science Education. University College of the Carib, Bachelor in Business Administration, B...
All SAT II Chemistry Resources
Popular Subjects
ISEE Tutors in Los Angeles, GMAT Tutors in Washington DC, Reading Tutors in Houston, Spanish Tutors in Boston, Calculus Tutors in Dallas Fort Worth, LSAT Tutors in Los Angeles, Reading Tutors in Phoenix, French Tutors in San Francisco-Bay Area, Biology Tutors in Chicago, SAT Tutors in Dallas Fort Worth
Popular Courses & Classes
GMAT Courses & Classes in Atlanta, SAT Courses & Classes in Miami, SSAT Courses & Classes in San Francisco-Bay Area, ISEE Courses & Classes in Atlanta, SAT Courses & Classes in Seattle, SSAT Courses & Classes in Los Angeles, LSAT Courses & Classes in Boston, GRE Courses & Classes in Houston, LSAT Courses & Classes in Chicago, Spanish Courses & Classes in Atlanta
Popular Test Prep
ACT Test Prep in Denver, ISEE Test Prep in Los Angeles, GMAT Test Prep in Atlanta, GMAT Test Prep in Phoenix, LSAT Test Prep in Seattle, SAT Test Prep in San Diego, GMAT Test Prep in Chicago, SAT Test Prep in Seattle, SAT Test Prep in San Francisco-Bay Area, ISEE Test Prep in New York City