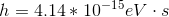All Physical Chemistry Resources
Example Questions
Example Question #1 : Photons And Photon Energy
Which of the following quantities is zero for photons?
Mass
Both mass and speed
Speed
Neither mass nor speed
Mass
Photons are fundamental particles that make up electromagnetic waves (light). The key aspect of photon is that it has very high speed 
Example Question #2 : Quantum Chemistry
Which of the following is/are the same for photons in gamma rays and in visible light?
I. frequency
II. wavelength
III. speed
I and II
I only
III only
None of these
III only
Electromagnetic spectrum is a spectrum of different types of light. Each type of electromagnetic wave differs from one another based on the frequency and wavelength; however, the speed of every electromagnetic wave in air is 
Here, 



Example Question #1 : Quantum Chemistry
Photons in ultraviolet rays have a wavelength of 10nm. What is the energy of photons in radio waves?
To solve this question, we need to use the equation relating energy of a wave to its wavelength.
Here, 




If we look at the electromagnetic spectrum we will notice that the wavelength of radio waves is a lot higher than ultraviolet rays. Since energy is inversely proportional to wavelength we can conclude that the energy of radio waves is lower than ultraviolet's. The only answer choice with an energy lower than ultraviolet rays energy is 1.24neV.
Certified Tutor
All Physical Chemistry Resources