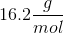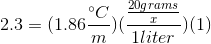All Physical Chemistry Resources
Example Questions
Example Question #2 : Colligative Properties
When 20 grams of a solute is added to a liter of water, the freezing point becomes 
Assume that the solute does not make ions in solution.

Possible Answers:
Correct answer:
Explanation:
We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.
Mehak
Certified Tutor
Certified Tutor
Guru Nanak Dev University, Bachelor of Science, Chemistry. Guru Nanak Dev University, Master of Science, Chemistry.
All Physical Chemistry Resources
Physical Chemistry Tutors in Top Cities:
Atlanta Physical Chemistry Tutors, Austin Physical Chemistry Tutors, Boston Physical Chemistry Tutors, Chicago Physical Chemistry Tutors, Dallas Fort Worth Physical Chemistry Tutors, Denver Physical Chemistry Tutors, Houston Physical Chemistry Tutors, Kansas City Physical Chemistry Tutors, Los Angeles Physical Chemistry Tutors, Miami Physical Chemistry Tutors, New York City Physical Chemistry Tutors, Philadelphia Physical Chemistry Tutors, Phoenix Physical Chemistry Tutors, San Diego Physical Chemistry Tutors, San Francisco-Bay Area Physical Chemistry Tutors, Seattle Physical Chemistry Tutors, St. Louis Physical Chemistry Tutors, Tucson Physical Chemistry Tutors, Washington DC Physical Chemistry Tutors
Popular Courses & Classes
SAT Courses & Classes in Los Angeles, MCAT Courses & Classes in Denver, SSAT Courses & Classes in Washington DC, SSAT Courses & Classes in Dallas Fort Worth, LSAT Courses & Classes in Miami, GMAT Courses & Classes in Atlanta, LSAT Courses & Classes in Philadelphia, MCAT Courses & Classes in Seattle, Spanish Courses & Classes in Boston, Spanish Courses & Classes in New York City