All Organic Chemistry Resources
Example Questions
Example Question #363 : Organic Chemistry
What is the arrangement of the substituents of the major product when bromine in iron (III) bromide reacts with bromobenzene?
None of these
Meta
Substituents will be on the same carbon
Para
Ortho
Para
When speaking of the arrangemts of two substituents on a benzene, their positions can be ortho, meta, or para. Ortho substituents are bound to adjacent carbons (C1 and C2). Meta substituents are bound to carbons with one intermediary carbon (C1 and C3). Para substituents are opposite one another across the benzene ring (C1 and C4).
The bromines in the given reaction result in a para product because bromine is ortho/para directing. There is a significant amount of ortho product, but because of steric hinderance, the major product is para.
Example Question #2 : Predicting Benzene Orientation

Suppose that toluene, whose chemical structure is shown, is reacted with 


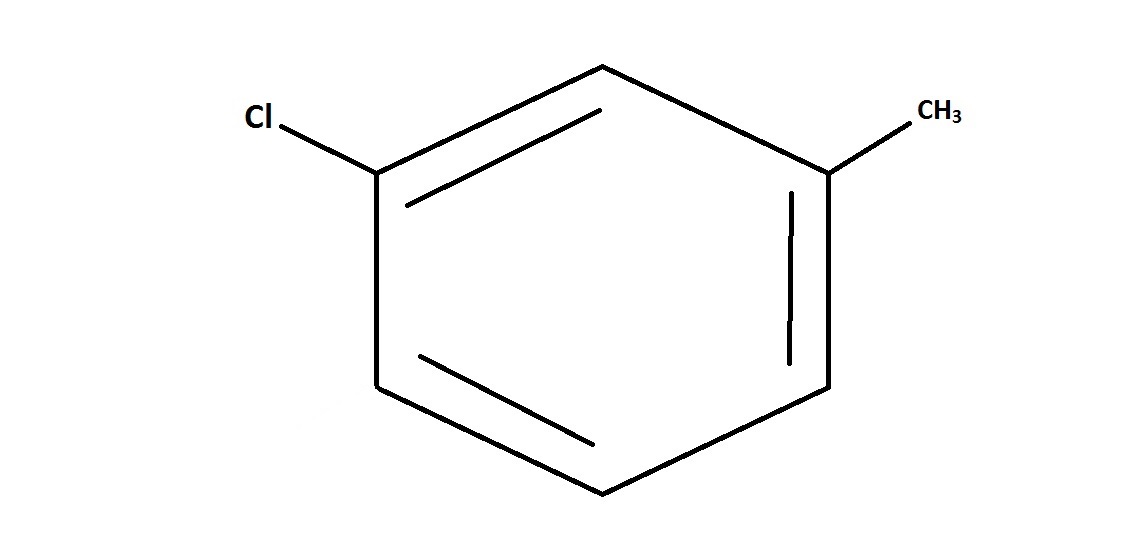



This question is essentially testing an understanding of electrophilic aromatic substitution and substituent placement. 


But we also need to be aware of what position this substituent will be directed to. Since the original molecule, toluene, consists of a benzene ring bonded to an electron-donating methyl group, this directs the substitution reaction towards the ortho or para position on the ring. Of the choices shown, only the para position is shown. The ortho position is not shown as an answer choice, and direction to the meta position would not be likely.
Example Question #13 : Benzene Additions

What is the product of the given reaction?






This is a trick question on Friedal-Craft alkylation. If you didn't pick the starting material, that is because you forgot the rule that alkylation cannot happen on a highly deactivated aromatic ring. Since 

Example Question #14 : Benzene Additions
What is the product for the following reaction?







While both the bromide and the alkyl group are ortho/para directing groups, the alkyl group is more activating and therefore the carbonyl will be added ortho to that substituent. The 


Example Question #371 : Organic Chemistry
Predict the product of the given reaction.

I
IV
II
III
IV
This is an example of a Friedel-Crafts acylation reaction, where an acyl group (an alkyl group with a carbonyl 

Example Question #371 : Organic Chemistry
What is the product of the reaction shown?

IV
I
II
III
II
This reaction is known as a nitration reaction, in which a nitro group (
All Organic Chemistry Resources




