All Organic Chemistry Resources
Example Questions
Example Question #1 : Reaction Mechanisms, Energetics, And Kinematics
Which of the following substrates would have the fastest reaction rate for an SN1 mechanism?
The SN1 mechanism involves the formation of a carbocation intermediate in the rate-determining step. The most stable carbocation will produce the fastest reaction. We can immediately eliminate any answer choices that will produce primary or secondary carbocations, since a tertiary carbocation will be much more stable. When comparing tertiary carbocations, larger and more electronegative substituents will allow for more charge stabilization.
Since the tertiary carbocation formed by the dissociation of iodide from 
Example Question #1 : Substitution Mechanisms
Which of the following determines the general rate of an 
Rate=k[nucleophile]
Rate=k[substrate][base]
Rate=k[substrate]
Rate=k[substrate][nucleophile]
Rate=k[substrate]
The rate of an 


Example Question #2 : Substitution Mechanisms
What is the final product of the pictured reaction?
1.
2.
3.
No reaction
Keep in mind that after the aldehyde is reduced into an alcohol, the molecule can undergo an intramolecular reaction, as alcohol is a good nucleophile and the halogen is a stellar leaving group.
Example Question #2 : Substitution Mechanisms
Which of the following is not true for an SN1 reaction?
Rearrangements are possible
Racemization of products
A strong nucleophile is required
All are true
A strong nucleophile is required
A strong nucleophile is not required for SN1. A weak nucleophile may be used. Remember that the SN1 mechanism goes through a carbocation intermediate.
Rearrangements are possible for SN1 reactions (not SN2). A rearrangement will occur to create a more stable intermediate in the mechanism. For example, if the carbocation is secondary, a methyl shift may occur to make the carbocation intermediate tertiary.
A racemic mixture of products occurs when with the nucleophile may attack the carbocation from either the top face or bottom face. When a reaction goes through a carbocation intermediate, as in SN1, there may be a racemic mix of products.
SN1 is unimolecular, and the rate of the reaction is determined by the substrate and reaction constant.
Example Question #3 : Substitution Mechanisms
A student carried out a substitution reaction in the lab using ethanol as a solvent. The student began with an optically pure reactant (100% (R)-configuration) and finished with a racemic mixture of products (50% (R)-configuration, 50% (S)-configuration).
The reaction went through which of the following mechanisms?
Either SN1 or SN2
SN2
E1
E2
SN1
SN1
SN1 reactions result in racemization when the nucleophile has a 50% chance of attacking the carbocation intermediate from the top face, and a 50% chance of attacking from the bottom face. SN1 reactions are favored in polar protic solvents, such as ethanol.
E2 and E1 are incorrect as they are elimination reaction mechanisms, and we are looking for a substitution mechanism. SN2 reactions result in inversion, not racemization. Additionally we know that SN2 is incorrect because SN2 is favored in polar aprotic solvents.
Certified Tutor
All Organic Chemistry Resources


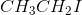


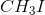








![\small Rate = k[Substrate]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/655607/gif.latex)



