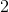All Organic Chemistry Resources
Example Questions
Example Question #71 : Organic Concepts
Which of the following is the correct major product of the above reaction?




Here we see an 
Example Question #1 : Help With Rearrangement Reactions
What is the major product of the reaction shown?


II
IV
I
III
V
IV
This reaction adds 


Example Question #51 : Reactions Types
What is the major product of the reaction shown?


IV
None of these
I
III
II
II
After carbocation is formed, a rearrangement reaction stabilizes positive charge by putting it on a tertiary carbon. This is done by a methyl shift. Recall that tertiary carbocations are the most stable due to the inductive effect of alkyl groups on the electron-deficient carbocation.
Example Question #71 : Organic Concepts
What is the major product of the reaction shown?
The first step of this reaction will be protonation of the hydroxyl oxygen to create a good leaving group. When the leaving group leaves, what's left is a secondary carbocation that is vicinal to (next to) a quaternary carbon. A methyl shift is thermodynamically favored in this case, as the rearrangement will leave a tertiary carbocation. Following the rearrangement the nucleophile (bromide) will attack the tertiary carbocation, forming a sigma bond with the carbon. The answer is thus 
Certified Tutor
All Organic Chemistry Resources