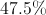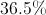All MCAT Physical Resources
Example Questions
Example Question #21 : Molecular Weight, Molecular Formula, And Moles
Manganese forms a number of oxides, one of which is composed of 72% manganese by mass. Which of the following is the formula for this oxide?
Given that the molar mass of oxygen is about 16g, and molar mass of manganese is about 55g, 
165g/229g = 0.72
So, the ratio of manganese to oxygen in this compound is 72% manganese by mass.
Example Question #22 : Molecular Weight, Molecular Formula, And Moles
What is the mass percentage of carbon in glucose (C6H12O6)?
96%
40%
25%
It depends on the amount of glucose.
40%
In order to find the mass percentage of an atom in a molecule, start by finding the total mass of one mole of the molecule. Glucose has 180 grams per mol.
Next, we determine the mass of the carbon atoms in one mole of the molecule. One carbon mole has a mass of 12 grams. Multiplied by the six carbon atoms in glucose gives a mass of 72 grams.
Finally, we divide the mass of carbon by the mass of the molecule.
So, 40% of glucose's mass is made up of carbon.
Example Question #23 : Molecular Weight, Molecular Formula, And Moles
What is the mass percentage of aluminum in aluminum (III) oxide?
Aluminum oxide has the formula 
Aluminum has a molecular weight of 

The mass percentage is given by the mass of aluminum divided by the total molecular weight.
Example Question #24 : Molecular Weight, Molecular Formula, And Moles
What is the percentage by weight of sodium in sodium sulfate?
Sodium sulfate is given by the formula:
To find the percentage by weight, we will need to divide the mass of sodium in the molecule by the total molecular mass.
Convert the ratio to a percentage.
Example Question #24 : Molecular Weight, Molecular Formula, And Moles
Which of the following samples contains a larger mass of hydrogen?
The samples contain equal amounts of hydrogen
We must know the density of each compound in order to solve
Start by calculating the percentages of hydrogen in each compound by using molar mass ratios.
Next, take the percentages of the given sample masses to determine the total mass of hydrogen in each.
We see that there is a larger mass of hydrogen in the hydrogen cyanide sample.
Example Question #23 : Molecular Weight, Molecular Formula, And Moles
How many grams of nitrogen are in 50g of ammonium sulfate?
12.3g
5.3g
11.6g
5.9g
10.6g
10.6g
First convert grams of ammonium sulfate to moles, then use the mole-to-mole ratio between nitrogen and ammonium sulfate. Finally, convert moles of nitrogen back into grams.
Example Question #27 : Molecular Weight, Molecular Formula, And Moles
What is the molecular weight of NaCl?
Molar mass of Na = 23g/mol
Molar mass of Cl = 35.5g/mol
58.5amu/mol
58.5amu
58.5g/mol
58.5g/amu
58.5amu
To find the molecular weight (mass) of a molecule, simply add up the atomic weights of each atom within the molecule. The units used will be amu (atomic mass units) for molecular weight and g/mol for molar mass.
Example Question #24 : Molecular Weight, Molecular Formula, And Moles
Convert 23g of water to moles.
1.4mol
0.8mol
1.3mol
2.6mol
1.3mol
First find the molar mass of water (H2O). You should be comfortable with the molar masses of hydrigen and oxygen from memory to reduce time on the MCAT exam.
Next, solve for moles.
Example Question #21 : Stoichiometry And Analytical Chemistry
Calculate the mass percent of sulfur in sulfuric acid 
To calculate the mass percent, calculate the the individual masses of each element. Then divide the mass of sulfur by the total mass of the molecule. Since there is only one sulfur atom in sulfuric acid, the mass of sulfur in one atom is 
Example Question #25 : Molecular Weight, Molecular Formula, And Moles
Compounds can be distinguished from each other by using their molecular weights. The molecular weight of a compound depends on the individual atomic weights of the elements and the amount of each element present in the compound. Consider hexane for example. Hexane has a molecular formula of 

A researcher is trying to determine the molecular formula of a hydrocarbon molecule. He measures the molecular weight to be 

The empirical formula for hydrocarbon is 










You can also calculate the ratio by simply looking at the empirical formula of this molecule (
Certified Tutor
Certified Tutor
All MCAT Physical Resources