All College Chemistry Resources
Example Questions
Example Question #1 : Calculating Solubility
What are the products of the following reaction?
The correct answer is
First of all, we know this is an acid-base reaction. 


Example Question #11 : Liquids And Solutions
Which of the following are soluble?
Chlorides
Carbonates
Hydroxides
Phosphates
Sulfides
Chlorides
Most salts containing halogens are soluble. Carbonates, phosphates, sulfides, oxides, and hydroxides are insoluble except for cations containing alkaline earth metals and hydroxides of calcium, strontium, and barium, which are slightly soluble.
Example Question #12 : Liquids And Solutions
Calculate the molar solubility of calcium hydroxide. Calcium hydroxide has a 

Start by writing the equation for the dissolution of calcium hydroxide.
Next, set up the following table to show the equilibrium concentrations of the ions:
|
|
[ |
[ |
|
Initial |
0.00 |
0.00 |
|
Change |
+S |
+2S |
|
Equilibrium |
S |
2S |
Now substitute in the values of the concentrations of the ions into the expression to find 
Now, plug in the given 

Example Question #21 : Liquids And Solutions
Consider an aqueous solution that is saturated with 
The magnesium concentration would need to be increased by a factor of
The magnesium concentration would need to be decreased by a factor of
The magnesium concentration would need to be increased by a factor of
The magnesium concentration would need to be decreased by a factor of
The magnesium concentration would need to be increased by a factor of
For this question, we're told that an aqueous solution of magnesium fluoride is saturated. We're then told that the fluoride concentration in the solution is reduced by a factor of two, and we're asked to find how the magnesium concentration would need to change in order to keep the solution saturated.
First, it's important to recall what saturation means. Some substances cannot dissolve in water, whereas others can dissolve readily. In other words, different substances will dissolve to differing degrees in water. The dissolution of a compound in a solvent such as water can be represented by an equilibrium expression. When there is a relatively small amount of solute added, the solution is said to be unsaturated. This means that all of the added solute will dissolve. As more and more solute is added to the water, there will eventually reach a point at which so much solute is present that it can no longer dissolve. When this happens, any additional solute will not dissolve and will instead form a precipitate in the solution. This condition is referred to as supersaturated. Saturation is the "sweet spot" so to speak; it is the point in between unsaturated and supersaturated where the maximum amount of solute has been added to the solution where all of the solute can be in the dissolved form. In other words, saturation refers to the maximum concentration of added solute where there is NO precipitation.
In order to set up an equilibrium expression, we can first write out the reaction in which magnesium fluoride dissolves.
Knowing the reaction, we can now write an equilibrium expression. Remember that pure solids and liquids don't appear in equilibrium expressions! Thus, this expression will only contain the products of the above reaction.
In the above expression, 
Since the equilibrium expression tells us the concentrations necessary to have a saturated solution, we can determine how a change in fluoride concentration would affect the equilibrium. Then, we can determine what changes to magnesium are needed.
If the fluoride concentration were to be cut in half, the 



Example Question #1 : Properties Of Gases
A gas does 


The correct answer is 



If a gas is expanding, it is doing work on the surroundings. This means that 

Example Question #1 : Gases
The graph shows molecular velocities for four different molecules at the same temperature. Which molecule has the highest molar mass?

A
B
D
C
A
Recall that the kinetic theory of gases states that if molecules are at the same temperature, then the mass of the molecules will determine their velocities. In a mixture of gases at a given temperature, the heavier gases will, on average, travel slower than the lighter ones.
Thus, A must be the gas with the highest molar mass because it has the most molecules traveling at the slowest velocity.
Example Question #32 : Solutions, States Of Matter, And Thermochemistry
Which is not characteristic of gases?
Gases are easy to compress.
The pressure of a gas decreases as more gas is added to the container.
Gases expand to fill their containers.
Gases occupy more space than the liquids or solids that they form.
The pressure of a gas decreases as more gas is added to the container.
There is more free space between gas particles than there is between liquid particles, and more free space between liquid particles than there is between solid particles. This makes gases very easy to compress as compared to solids and liquids.
Both liquids and gases can expand to fill their containers because the individual particles can move past each other. This is not possible in a solid because the individual particles are rigidly packed.
Additionally, the volume of a liquid or solid can increase by approximately eight hundred times when it becomes a gas. This large change in volume can be harnessed to do work. For example, a steam engine works when water boils to form a gas (steam), which has a larger volume. Steam can thus escape from the container in which it was produced and perform work.
Lastly, the pressure of a gas increases as more gas is added to the container. Pressure is defined as the force exerted by the gas over a certain area. If more gas particles are added to a fixed area, they will exert a greater force over that area, increasing the overall pressure.
Example Question #1 : Properties Of Gases
The Haber process is a common reaction used in the industrial production of ammonia:
Which of the following would increase the production of NH3(g)?
Adding water
Increasing volume
Adding an inert gas
Decreasing volume
Increasing temperature
Decreasing volume
Let's first look at the affects of adding an inert gas such as 
Since He is on both sides of the equation, equilibrium is not affected.
Now let's look at the affects of heat. Because the reaction is exothermic, it can be rewritten as
Since heat is a product of the reaction, increasing temperature would shift the reaction to the left.
Finally, we know that changing the volume changes the internal pressure of the system. We know that the affect of this change is dependent on the number of moles in the gaseous phase.
In order to increase the production of NH3 (the side with the least number of moles), we need to increase the internal pressure of the system by decreasing the volume.
Decreasing the volume would increase the production of ammonia.
Example Question #1 : Non Ideal Gas Behavior
One flask is at STP and another is at 
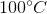
The pressure of the flask at 

Because the volume between the flasks and the moles in each flask are constant, we can cancel out 

At STP, conditions are 

Example Question #1 : Non Ideal Gas Behavior
A 3.00 L container at 273 K is filled with 1.00 mol Cl2(g), which behaves non-ideally.
Using the van der Waals equation, calculate pressure exerted by the gas.

Recall the van der Waals equation for non-ideal gases
Rewrite the equation using the known values and solve for P
All College Chemistry Resources













 ]
] ]
]![\text{K}_{sp}=[\text{Ca}^{2+}][\text{OH}^-]^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/902741/gif.latex)









![K_{sp}=[Mg^{2+}][F^{-}]^2](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1006544/gif.latex)
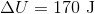

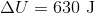

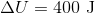






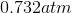
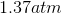

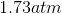



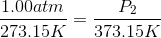


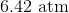




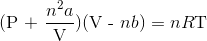
![[\textrm{P + }\frac{(1\textrm{ mol})^{2}(6.49\frac{\textrm{L}^{2}\textrm{ atm}}{\textrm{mol}^{2}})}{(3.00\textrm{ L})^2}][\textrm{3.00 L - (1 mol)}(0.0562\frac{\textrm{L}}{\textrm{mol}})]=(1\textrm{ mol})^{2}(0.0821\frac{\textrm{atm}}{\textrm{mol K}})(273K)](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/996927/gif.latex)





