Carbonyl Products - Organic Chemistry
Card 0 of 88

What is the product when the given starting compound is reacted with lithium aluminum hydride and acid?

What is the product when the given starting compound is reacted with lithium aluminum hydride and acid?
Tap to see back →
This reaction involves a very strong reducing agent in lithium aluminum hydride,  . LAH converts ketones, aldehydes, esters, and acid chlorides into alcohols. This reaction changes the carbonyl group into a hydroxyl group. As a result, the final answer is 2-butanol.
. LAH converts ketones, aldehydes, esters, and acid chlorides into alcohols. This reaction changes the carbonyl group into a hydroxyl group. As a result, the final answer is 2-butanol.
This reaction involves a very strong reducing agent in lithium aluminum hydride, 
What reagents are required to efficiently form a tertiary alcohol with two of the same substituents in only two steps?
What reagents are required to efficiently form a tertiary alcohol with two of the same substituents in only two steps?
Tap to see back →
This is an example of a Grignard reaction that requires the use of an ester or acid chloride, and more than one equivalent of an organometallic. The second step would be to react the product of the first step in an acidic source.
This is an example of a Grignard reaction that requires the use of an ester or acid chloride, and more than one equivalent of an organometallic. The second step would be to react the product of the first step in an acidic source.
What reagents are required to undergo Swern oxidation?
What reagents are required to undergo Swern oxidation?
Tap to see back →
Swern oxidation requires COCl2, triethylamine, and DMSO. The same reaction can be accomplished using PCC and dichloromethane. This reaction oxidizes an alcohol to a carbonyl, but only works with primary and secondary alcohols.
Swern oxidation requires COCl2, triethylamine, and DMSO. The same reaction can be accomplished using PCC and dichloromethane. This reaction oxidizes an alcohol to a carbonyl, but only works with primary and secondary alcohols.

Which of the following reactions will result in the given product?

Which of the following reactions will result in the given product?
Tap to see back →
The starting material first needs to be brominated, which occur at the tertiary carbon. Then, an elimination reaction needs to take place to form an alkene intermediate, which will transpire through the E2 pathway with the tertbutoxide ion in tertbutanol. After this, an alcohol needs to be formed through hydroboration because we need the carbonyl group on the less subsituted carbon. After an alcohol is formed, the PCC reaction can oxidize the alcohol to make it a carbonyl. All these reactions are shown in choice C.
The starting material first needs to be brominated, which occur at the tertiary carbon. Then, an elimination reaction needs to take place to form an alkene intermediate, which will transpire through the E2 pathway with the tertbutoxide ion in tertbutanol. After this, an alcohol needs to be formed through hydroboration because we need the carbonyl group on the less subsituted carbon. After an alcohol is formed, the PCC reaction can oxidize the alcohol to make it a carbonyl. All these reactions are shown in choice C.
Which of the following reagents is needed to convert an amide into a carboxylic acid?
Which of the following reagents is needed to convert an amide into a carboxylic acid?
Tap to see back →
As a general rule, any carboxylic acid derivative can be converted into al carboxylic acid when reacting with 
As a general rule, any carboxylic acid derivative can be converted into al carboxylic acid when reacting with

What is the product of the given reaction?

What is the product of the given reaction?
Tap to see back →
The hydrolyzation of a nitrile with hydronium leads to a carboxylic acid. Only one choice is a carboxylic acid.
The hydrolyzation of a nitrile with hydronium leads to a carboxylic acid. Only one choice is a carboxylic acid.
What is the product of a hydroboration–oxidation reaction with 1-hexylcyclohexene?
What is the product of a hydroboration–oxidation reaction with 1-hexylcyclohexene?
Tap to see back →
This reaction is an electrophilic addition reaction with an alkene. This is one of many alkene addition reactions that can add an -OH group onto your starting material. The key aspect of an hydroboration-oxidation reaction is the anti-Markovinikov addition to the double bond. The -OH group should be on the least substituted of the two carbons that originate from the double bond. In light of this information, the answer is 2-cyclohexanol.
This reaction is an electrophilic addition reaction with an alkene. This is one of many alkene addition reactions that can add an -OH group onto your starting material. The key aspect of an hydroboration-oxidation reaction is the anti-Markovinikov addition to the double bond. The -OH group should be on the least substituted of the two carbons that originate from the double bond. In light of this information, the answer is 2-cyclohexanol.
A compound,  , is reacted with sodium ethoxide to give the single elimination product
, is reacted with sodium ethoxide to give the single elimination product  . This product then reacts with ozone, zinc, and water to give the product shown below.
. This product then reacts with ozone, zinc, and water to give the product shown below.

What is the original compound?
A compound, 


What is the original compound?
Tap to see back →
In the first step of the reaction, a chlorine is abstracted and a double bond is formed. In the ozonolysis step, the molecule is broken at the double bond and each carbon at that bond gets double bonded to an oxygen. The presence of zinc keeps it from becoming a carboxylic acid.
Working backwards, you must remove the oxygens from the final product and redraw the molecule at the double bond. This intermediate is 1,3-dimethylcyclopentene. Next, the chlorine must be added to one of the carbons on the double bond. The only possible choice is 2-chloro-1,3-dimethylcyclopentane.
In the first step of the reaction, a chlorine is abstracted and a double bond is formed. In the ozonolysis step, the molecule is broken at the double bond and each carbon at that bond gets double bonded to an oxygen. The presence of zinc keeps it from becoming a carboxylic acid.
Working backwards, you must remove the oxygens from the final product and redraw the molecule at the double bond. This intermediate is 1,3-dimethylcyclopentene. Next, the chlorine must be added to one of the carbons on the double bond. The only possible choice is 2-chloro-1,3-dimethylcyclopentane.

Which of the following reagents is required to produce the ketone product?

Which of the following reagents is required to produce the ketone product?
Tap to see back →
In order to convert a secondary alcohol into a ketone, we must employ  as a reagent.
as a reagent.
In order to convert a secondary alcohol into a ketone, we must employ 
Which of the following reactions would NOT produce a carboxylic acid?
Which of the following reactions would NOT produce a carboxylic acid?
Tap to see back →
PCC is considered a weak oxidizing agent. The reaction of a primary alcohol with PCC would only yield an aldehyde, while reaction with a secondary alcohol will yield a ketone. PCC will not be used to generate carboxylic acids.
A stronger oxidation, like  or
or  , is required to oxidize up to the carboxylic acid. Treatment of an ester with a base or treatment of carbon dioxide with a Grignard reagent are other ways of making carboxylic acids.
, is required to oxidize up to the carboxylic acid. Treatment of an ester with a base or treatment of carbon dioxide with a Grignard reagent are other ways of making carboxylic acids.
PCC is considered a weak oxidizing agent. The reaction of a primary alcohol with PCC would only yield an aldehyde, while reaction with a secondary alcohol will yield a ketone. PCC will not be used to generate carboxylic acids.
A stronger oxidation, like 

Which one of the following compounds can produce a carboxylic acid only when reacted with sodium dichromate and sulfuric acid?
Which one of the following compounds can produce a carboxylic acid only when reacted with sodium dichromate and sulfuric acid?
Tap to see back →
After recognizing the reagents given, we know we need to begin with an alcohol. The alcohol choices differ only by how substituted they are.
For a carboxylic acid to form from a reaction with sodium dichromate and sulfuric acid, a primary alcohol needs to be available. Therefore, 1-pentanol is the correct answer.
After recognizing the reagents given, we know we need to begin with an alcohol. The alcohol choices differ only by how substituted they are.
For a carboxylic acid to form from a reaction with sodium dichromate and sulfuric acid, a primary alcohol needs to be available. Therefore, 1-pentanol is the correct answer.
When 3-chloroheptane undergoes malonic ester synthesis, the final product is .
When 3-chloroheptane undergoes malonic ester synthesis, the final product is .
Tap to see back →
The malonic ester is deprotonated at the most acidic hydrogen, the one on the carbon between the two oxygens. The electrons from that bond to the hydrogen form a carbon-carbon double bond while electrons from the oxygen-carbon double bond go to the oxygen atom. This is the enolate form. The chlorine leaves the 3-chloroheptane and the electrons from the carbon-carbon double bond bond to the carbon that the chlorine left. At the same time, the carbonyl is reformed. After deesterfication, 3-ethylheptanoic acid is formed.
The malonic ester is deprotonated at the most acidic hydrogen, the one on the carbon between the two oxygens. The electrons from that bond to the hydrogen form a carbon-carbon double bond while electrons from the oxygen-carbon double bond go to the oxygen atom. This is the enolate form. The chlorine leaves the 3-chloroheptane and the electrons from the carbon-carbon double bond bond to the carbon that the chlorine left. At the same time, the carbonyl is reformed. After deesterfication, 3-ethylheptanoic acid is formed.
Which of the following is not a derivative of a carboxylic acid?
Which of the following is not a derivative of a carboxylic acid?
Tap to see back →
Aldehyde is not a derivative of carboxylic acid. Esters can be derived from carboxylic acids by reacting them with  to form an acid chloride. From there, react it with an
to form an acid chloride. From there, react it with an  to form an ester. Amides can be derived from carboxylic acids by reacting them with
to form an ester. Amides can be derived from carboxylic acids by reacting them with  to form an acid chloride. From there, react it with
to form an acid chloride. From there, react it with  to form an amide.
to form an amide.
Aldehyde is not a derivative of carboxylic acid. Esters can be derived from carboxylic acids by reacting them with 



Please choose the best answer for the following question.
Which of the following reagents is best for converting a primary alcohol to a carboxylic acid?
Please choose the best answer for the following question.
Which of the following reagents is best for converting a primary alcohol to a carboxylic acid?
Tap to see back →
 is the only strong oxidizing agent listed. It is strong enough to oxidize the primary alcohol even further to a carboxylic acid product. The rest of the compounds listed are weak oxidizing agents or reducing agents.
is the only strong oxidizing agent listed. It is strong enough to oxidize the primary alcohol even further to a carboxylic acid product. The rest of the compounds listed are weak oxidizing agents or reducing agents.

Which of the following reactions is NOT a valid synthesis of methyl benzoate?
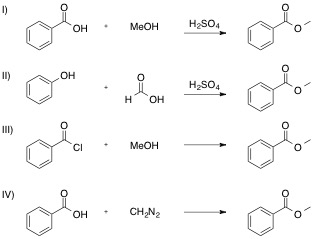
Which of the following reactions is NOT a valid synthesis of methyl benzoate?

Tap to see back →
Each reaction is shown, with its name and correct product below.
If you are unsure how the product is obtained through each reaction, use your textbook or internet sources to find a mechanism for each. Reactions I, II, and III are specific examples of substitution reactions, and the last, reaction IV, goes through a more complex addition-elimination reaction to release nitrogen gas.
Working through the mechanism for a reaction is always a certain way to find the product, even if you don't know from the outset what it will be. Just look for the electrophile and nucleophile in each step of the mechanism, and push those arrows!

Each reaction is shown, with its name and correct product below.
If you are unsure how the product is obtained through each reaction, use your textbook or internet sources to find a mechanism for each. Reactions I, II, and III are specific examples of substitution reactions, and the last, reaction IV, goes through a more complex addition-elimination reaction to release nitrogen gas.
Working through the mechanism for a reaction is always a certain way to find the product, even if you don't know from the outset what it will be. Just look for the electrophile and nucleophile in each step of the mechanism, and push those arrows!


What reagent is required to complete the reaction?

What reagent is required to complete the reaction?
Tap to see back →
This reaction is completed by tosylating the hydroxyl group to make it a good leaving group (keep in mind the stereochemistry is retained through the tosylation reaction). The second step is an SN2 reaction with the methoxide ion, which gives the correct stereochemistry. HBr will not result in the correct stereochemical product.
This reaction is completed by tosylating the hydroxyl group to make it a good leaving group (keep in mind the stereochemistry is retained through the tosylation reaction). The second step is an SN2 reaction with the methoxide ion, which gives the correct stereochemistry. HBr will not result in the correct stereochemical product.
What is the product of the reaction between sodium ethoxide and 1-bromopropane?
What is the product of the reaction between sodium ethoxide and 1-bromopropane?
Tap to see back →
In solution, the sodium in sodium ethoxide will ionize and an  ion will be present.
ion will be present.
Bromine is a good leaving group. In the same step, the bromine leaves and the electrons from the ethoxide will attack that carbon. This creates a bond between the carbon and an oxygen.
The final product is an ether with three carbons on one side and two on the other. This is 1-ethoxypropane.
In solution, the sodium in sodium ethoxide will ionize and an 
Bromine is a good leaving group. In the same step, the bromine leaves and the electrons from the ethoxide will attack that carbon. This creates a bond between the carbon and an oxygen.
The final product is an ether with three carbons on one side and two on the other. This is 1-ethoxypropane.
An organic chemist wants to synthesize an ether product. She begins with methanol and ethanol as her substrates. How would she go about synthesizing the desired ether product?
An organic chemist wants to synthesize an ether product. She begins with methanol and ethanol as her substrates. How would she go about synthesizing the desired ether product?
Tap to see back →
The substrates must be modified in order to attain the desired product. In order to get the desired product, she needs to change one of the alcohol groups into a good leaving group. One way to do this is to react the methanol with  to create a primary tosylate. Because of its advanced resonance, tosylate is an extraordinary leaving group, and so the ethanol is free to attack the modified substrate to form an ether.
to create a primary tosylate. Because of its advanced resonance, tosylate is an extraordinary leaving group, and so the ethanol is free to attack the modified substrate to form an ether.
The substrates must be modified in order to attain the desired product. In order to get the desired product, she needs to change one of the alcohol groups into a good leaving group. One way to do this is to react the methanol with 
Consider the following reaction.




In this reaction, two molecules of an alpha-hydroxy acid are condensed with heat to form product. What functional group is created in this reaction?
Consider the following reaction.


In this reaction, two molecules of an alpha-hydroxy acid are condensed with heat to form product. What functional group is created in this reaction?
Tap to see back →
For this question, we're shown the structures of both the reactants and products, and we're asked to identify which functional group is in the product.
First, let's go through the answer choices and define them. Then, we'll compare that definition with what we see in the product.
Aldehydes are functional groups in which a carbon atom is double bonded to an oxygen atom. The carbon atom must also be bound to at least one hydrogen atom, and to another hydrogen or carbon atom. Usually, aldehydes occur at the terminal ends of a molecule.
Ketones are similar to aldehydes. These functional groups contain a carbon atom double bound to an oxygen atom. In addition, the carbon atom is bound to two other carbon atoms. Thus, ketones tend to be found within a compound, as opposed to at the terminal ends.
Ethers are a class of functional group in which an oxygen atom is situated between two carbon atoms via a single bond.
Carboxylic acids are a functional group in which a carbon is double bound to one oxygen atom, and also single bonded to the oxygen of a hydroxyl group. This functional group does not occur in the product, but it does occur in the reactant.
Esters are a functional group in which a carbon atom is double bonded to an oxygen atom, and also single bonded to another oxygen atom. However, unlike carboxylic acids, esters do not contain a hydroxyl group. Instead, the oxygen atom is bound to another "R group," typically another carbon atom.
Now, let's go ahead and take a look at the product. Shown in red circles, we see that there are two ester groups in the product. Thus, the ester functional group is the correct answer.

For this question, we're shown the structures of both the reactants and products, and we're asked to identify which functional group is in the product.
First, let's go through the answer choices and define them. Then, we'll compare that definition with what we see in the product.
Aldehydes are functional groups in which a carbon atom is double bonded to an oxygen atom. The carbon atom must also be bound to at least one hydrogen atom, and to another hydrogen or carbon atom. Usually, aldehydes occur at the terminal ends of a molecule.
Ketones are similar to aldehydes. These functional groups contain a carbon atom double bound to an oxygen atom. In addition, the carbon atom is bound to two other carbon atoms. Thus, ketones tend to be found within a compound, as opposed to at the terminal ends.
Ethers are a class of functional group in which an oxygen atom is situated between two carbon atoms via a single bond.
Carboxylic acids are a functional group in which a carbon is double bound to one oxygen atom, and also single bonded to the oxygen of a hydroxyl group. This functional group does not occur in the product, but it does occur in the reactant.
Esters are a functional group in which a carbon atom is double bonded to an oxygen atom, and also single bonded to another oxygen atom. However, unlike carboxylic acids, esters do not contain a hydroxyl group. Instead, the oxygen atom is bound to another "R group," typically another carbon atom.
Now, let's go ahead and take a look at the product. Shown in red circles, we see that there are two ester groups in the product. Thus, the ester functional group is the correct answer.

When butanoic acid undergoes the Hell-Volhard-Zelinsky (HVZ) reaction, the final product is .
When butanoic acid undergoes the Hell-Volhard-Zelinsky (HVZ) reaction, the final product is .
Tap to see back →
In the HVZ reaction of a carboxylic acid, a bromine is added to the alpha carbon. Phosphorus catalyzes the reaction and allows for the formation of an acyl halide. Acyl halides readily undergo enol-ketone tautomerization. The enol form uses electrons from the carbon-carbon double bond to bond to the bromine. In water, the two bromine molecules convert back to a carboxylic acid with one bromine on the alpha carbon.
In the HVZ reaction of a carboxylic acid, a bromine is added to the alpha carbon. Phosphorus catalyzes the reaction and allows for the formation of an acyl halide. Acyl halides readily undergo enol-ketone tautomerization. The enol form uses electrons from the carbon-carbon double bond to bond to the bromine. In water, the two bromine molecules convert back to a carboxylic acid with one bromine on the alpha carbon.