Mechanisms and Intermediates - GRE
Card 0 of 64
Which of the following transformations includes an enolate intermediate?
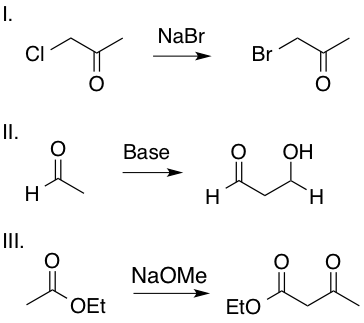
Which of the following transformations includes an enolate intermediate?

Enolates are formed by an oxygen anion bound to an alkene carbon. Reactions II and III include an enolate intermediate, as shown in the mechanisms below, whereas reaction I is a simple SN2 reaction and does not include an enolate intermediate. Enolates are highlighted in red.

Enolates are formed by an oxygen anion bound to an alkene carbon. Reactions II and III include an enolate intermediate, as shown in the mechanisms below, whereas reaction I is a simple SN2 reaction and does not include an enolate intermediate. Enolates are highlighted in red.

Compare your answer with the correct one above
Which of the following would be the best base to perform an E2 elimination?
Which of the following would be the best base to perform an E2 elimination?
The correct answer is sodium butoxide. Remember from your studies that for E2 reactions to be highly preferred they require a "big, bulky, base".
First eliminate ammonium 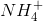 for it is not a base and is the conjugate acid form of ammonia. Then we can eliminate methanol (it is not in its conjugate base form) because there are stronger bases among the answer choices.
for it is not a base and is the conjugate acid form of ammonia. Then we can eliminate methanol (it is not in its conjugate base form) because there are stronger bases among the answer choices.
Now we can look at the real bases. Sodium cyanide will yield the cyanide ion, which is an extremely strong nucleophile. Remember that the E2 competes with the SN2. This means that the cyanide ion will prefer performing the SN2 not the E2, so we can cross this answer choice off.
Finally we are down to 2 answer choices, potassium hydroxide and sodium butoxide. Though potassium hydroxide is a strong base, it does not come close to the bulkiness of sodium butoxide. Thus, our best choice is sodium butoxide.
The correct answer is sodium butoxide. Remember from your studies that for E2 reactions to be highly preferred they require a "big, bulky, base".
First eliminate ammonium 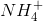
Now we can look at the real bases. Sodium cyanide will yield the cyanide ion, which is an extremely strong nucleophile. Remember that the E2 competes with the SN2. This means that the cyanide ion will prefer performing the SN2 not the E2, so we can cross this answer choice off.
Finally we are down to 2 answer choices, potassium hydroxide and sodium butoxide. Though potassium hydroxide is a strong base, it does not come close to the bulkiness of sodium butoxide. Thus, our best choice is sodium butoxide.
Compare your answer with the correct one above
Which of the following carbocation intermediates requires the least activation energy?
Which of the following carbocation intermediates requires the least activation energy?
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
Compare your answer with the correct one above
What intermediate is involved in the conversion of compound B to compound C?

What intermediate is involved in the conversion of compound B to compound C?

The strong sulfuric acid protonates the hydroxyl group of compound B, resulting in the loss of water as a leaving group and the generation of a carbocation intermediate. Since this carbocation carbon is attached to three other carbons, this is a tertiary carbocation. It is bound to the phenyl substituent, a methyl group, and the branched carbon chain.
The strong sulfuric acid protonates the hydroxyl group of compound B, resulting in the loss of water as a leaving group and the generation of a carbocation intermediate. Since this carbocation carbon is attached to three other carbons, this is a tertiary carbocation. It is bound to the phenyl substituent, a methyl group, and the branched carbon chain.
Compare your answer with the correct one above
Carbon 1:

Carbon 2:

Let's say we react the given compound with  . During the first step of the reaction, will the hydrogen be added to carbon 1 or carbon 2, why?
. During the first step of the reaction, will the hydrogen be added to carbon 1 or carbon 2, why?
Carbon 1:

Carbon 2:

Let's say we react the given compound with 
The correct answer is: carbon 1 because the intermediate will then be a secondary carbocation.
This is a case of  addition across a double bond where
addition across a double bond where  stands for any halide. The first step in this reaction is the attack of
stands for any halide. The first step in this reaction is the attack of  by the double bond. This will create two intermediates, the first being the halide anion
by the double bond. This will create two intermediates, the first being the halide anion  (so in our case
(so in our case  ), the second being a carbocation on our compound at one of the two carbons that formerly shared the double bond.
), the second being a carbocation on our compound at one of the two carbons that formerly shared the double bond.
If the hydrogen attached the carbon 2 then we would have a positive charge on carbon 1 and vice versa. A positive charge on carbon 1 is known as a primary carbocation (a carbon attached to 1 other carbon or function group), which is rarely if ever seen due to its overwhelming instability. A positive charge on carbon 2 is known as a secondary carbocation (a carbon attached to 2 other carbons or functional groups) and is much more stable than a primary carbocation.
We would want the more thermodynamically stable intermediate for our reaction to proceed, so we would want the positive charge on carbon 2 and the hydrogen attached to carbon 1.
The correct answer is: carbon 1 because the intermediate will then be a secondary carbocation.
This is a case of 




If the hydrogen attached the carbon 2 then we would have a positive charge on carbon 1 and vice versa. A positive charge on carbon 1 is known as a primary carbocation (a carbon attached to 1 other carbon or function group), which is rarely if ever seen due to its overwhelming instability. A positive charge on carbon 2 is known as a secondary carbocation (a carbon attached to 2 other carbons or functional groups) and is much more stable than a primary carbocation.
We would want the more thermodynamically stable intermediate for our reaction to proceed, so we would want the positive charge on carbon 2 and the hydrogen attached to carbon 1.
Compare your answer with the correct one above

If the carbon being pointed to was deprotonated (resulting in a positive charge on it). Would the resonance form (the positive charge being redistributed to the carbon with a bromine) be more stable than a secondary carbocation? Why?

If the carbon being pointed to was deprotonated (resulting in a positive charge on it). Would the resonance form (the positive charge being redistributed to the carbon with a bromine) be more stable than a secondary carbocation? Why?
The resonance form of this compound would put the positive charge on the carbon attached to the bromine. Unfortunately this carbon is already slightly positive due to the electron withdrawing effects of bromine due to its high electrophilicity. So this resonance form would be more unstable than a secondary carbocation due to the increased concentration of positive charge from bromine's electron withdrawal.
The resonance form of this compound would put the positive charge on the carbon attached to the bromine. Unfortunately this carbon is already slightly positive due to the electron withdrawing effects of bromine due to its high electrophilicity. So this resonance form would be more unstable than a secondary carbocation due to the increased concentration of positive charge from bromine's electron withdrawal.
Compare your answer with the correct one above
When exposed to a good nucleophile, which molecule will most readily undergo an  reaction?
reaction?
When exposed to a good nucleophile, which molecule will most readily undergo an 
 reactions, also known as unimolecular nucleophilic substitution reactions, occur in two steps. Here, we are concerned with the first and second (rate-determining) steps, in which the leaving group breaks off of the molecule to form a carbocation. Alkanes that form the most stable carbocations are most likely to undergo
reactions, also known as unimolecular nucleophilic substitution reactions, occur in two steps. Here, we are concerned with the first and second (rate-determining) steps, in which the leaving group breaks off of the molecule to form a carbocation. Alkanes that form the most stable carbocations are most likely to undergo 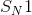 reactions. Tertiary carbocations are the most stable, followed by secondary. Primary and methyl carbocations are very unstable and unlikely to form at all. The tertiary alkane,
reactions. Tertiary carbocations are the most stable, followed by secondary. Primary and methyl carbocations are very unstable and unlikely to form at all. The tertiary alkane,  , will form a very stable tertiary carbocation compared to the other answer choices.
, will form a very stable tertiary carbocation compared to the other answer choices.

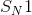

Compare your answer with the correct one above

For which of the following acid-base reactions will the equilibrium lie on the left side?
For which of the following acid-base reactions will the equilibrium lie on the left side?
The pKa value indicates how strong an acid is, and acid strength increases as pKa decreases. The side of a reaction with a lower pKa is going to dissociate more, pushing the equilibrium over to the other side. The equilibrium will thus lie on the side with the HIGHER pKa.

Since the pKa of acetic acid (4.76) is higher than the pKa of trifluoroacetic acid (0), the reaction will shift to the left to reach equilibrium.
The pKa value indicates how strong an acid is, and acid strength increases as pKa decreases. The side of a reaction with a lower pKa is going to dissociate more, pushing the equilibrium over to the other side. The equilibrium will thus lie on the side with the HIGHER pKa.
Since the pKa of acetic acid (4.76) is higher than the pKa of trifluoroacetic acid (0), the reaction will shift to the left to reach equilibrium.
Compare your answer with the correct one above
Which of the following carbocation intermediates requires the least activation energy?
Which of the following carbocation intermediates requires the least activation energy?
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
Compare your answer with the correct one above
What intermediate is involved in the conversion of compound B to compound C?

What intermediate is involved in the conversion of compound B to compound C?

The strong sulfuric acid protonates the hydroxyl group of compound B, resulting in the loss of water as a leaving group and the generation of a carbocation intermediate. Since this carbocation carbon is attached to three other carbons, this is a tertiary carbocation. It is bound to the phenyl substituent, a methyl group, and the branched carbon chain.
The strong sulfuric acid protonates the hydroxyl group of compound B, resulting in the loss of water as a leaving group and the generation of a carbocation intermediate. Since this carbocation carbon is attached to three other carbons, this is a tertiary carbocation. It is bound to the phenyl substituent, a methyl group, and the branched carbon chain.
Compare your answer with the correct one above
Carbon 1:

Carbon 2:

Let's say we react the given compound with  . During the first step of the reaction, will the hydrogen be added to carbon 1 or carbon 2, why?
. During the first step of the reaction, will the hydrogen be added to carbon 1 or carbon 2, why?
Carbon 1:

Carbon 2:

Let's say we react the given compound with 
The correct answer is: carbon 1 because the intermediate will then be a secondary carbocation.
This is a case of  addition across a double bond where
addition across a double bond where  stands for any halide. The first step in this reaction is the attack of
stands for any halide. The first step in this reaction is the attack of  by the double bond. This will create two intermediates, the first being the halide anion
by the double bond. This will create two intermediates, the first being the halide anion  (so in our case
(so in our case  ), the second being a carbocation on our compound at one of the two carbons that formerly shared the double bond.
), the second being a carbocation on our compound at one of the two carbons that formerly shared the double bond.
If the hydrogen attached the carbon 2 then we would have a positive charge on carbon 1 and vice versa. A positive charge on carbon 1 is known as a primary carbocation (a carbon attached to 1 other carbon or function group), which is rarely if ever seen due to its overwhelming instability. A positive charge on carbon 2 is known as a secondary carbocation (a carbon attached to 2 other carbons or functional groups) and is much more stable than a primary carbocation.
We would want the more thermodynamically stable intermediate for our reaction to proceed, so we would want the positive charge on carbon 2 and the hydrogen attached to carbon 1.
The correct answer is: carbon 1 because the intermediate will then be a secondary carbocation.
This is a case of 




If the hydrogen attached the carbon 2 then we would have a positive charge on carbon 1 and vice versa. A positive charge on carbon 1 is known as a primary carbocation (a carbon attached to 1 other carbon or function group), which is rarely if ever seen due to its overwhelming instability. A positive charge on carbon 2 is known as a secondary carbocation (a carbon attached to 2 other carbons or functional groups) and is much more stable than a primary carbocation.
We would want the more thermodynamically stable intermediate for our reaction to proceed, so we would want the positive charge on carbon 2 and the hydrogen attached to carbon 1.
Compare your answer with the correct one above

If the carbon being pointed to was deprotonated (resulting in a positive charge on it). Would the resonance form (the positive charge being redistributed to the carbon with a bromine) be more stable than a secondary carbocation? Why?

If the carbon being pointed to was deprotonated (resulting in a positive charge on it). Would the resonance form (the positive charge being redistributed to the carbon with a bromine) be more stable than a secondary carbocation? Why?
The resonance form of this compound would put the positive charge on the carbon attached to the bromine. Unfortunately this carbon is already slightly positive due to the electron withdrawing effects of bromine due to its high electrophilicity. So this resonance form would be more unstable than a secondary carbocation due to the increased concentration of positive charge from bromine's electron withdrawal.
The resonance form of this compound would put the positive charge on the carbon attached to the bromine. Unfortunately this carbon is already slightly positive due to the electron withdrawing effects of bromine due to its high electrophilicity. So this resonance form would be more unstable than a secondary carbocation due to the increased concentration of positive charge from bromine's electron withdrawal.
Compare your answer with the correct one above
Which substance is used as a reducing agent?
Which substance is used as a reducing agent?
A reducing agent is a substance that readily donates an electron to another substance. They consist primarily of elements with low electronegativities such as hydrogen. Therefore, the answer is 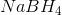 (sodium borohydride) which consists primarily of hydrogen atoms.
(sodium borohydride) which consists primarily of hydrogen atoms.
A reducing agent is a substance that readily donates an electron to another substance. They consist primarily of elements with low electronegativities such as hydrogen. Therefore, the answer is 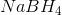
Compare your answer with the correct one above
In reactions involving the alkylation of acetylide ions, it is preferred that the alkyl halide be primary. What is the reason for this?
In reactions involving the alkylation of acetylide ions, it is preferred that the alkyl halide be primary. What is the reason for this?
The reason that the alkyl halide is preferred to be primary is because the mechanism for these reactions is SN2. SN2 indicates a substitution reaction that takes place in one step. A primary alcohol is preferred to prevent steric congestion caused by the simultaneous binding of the nucleophile and release of the leaving group. This reaction mechanism is faster because it omits the formation of a carbocation intermediate.
In contrast, SN1 reactions take place in two steps and involve the formation of a carbocation intermediate.
The reason that the alkyl halide is preferred to be primary is because the mechanism for these reactions is SN2. SN2 indicates a substitution reaction that takes place in one step. A primary alcohol is preferred to prevent steric congestion caused by the simultaneous binding of the nucleophile and release of the leaving group. This reaction mechanism is faster because it omits the formation of a carbocation intermediate.
In contrast, SN1 reactions take place in two steps and involve the formation of a carbocation intermediate.
Compare your answer with the correct one above
Which of the following carbocation intermediates requires the least activation energy?
Which of the following carbocation intermediates requires the least activation energy?
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
Compare your answer with the correct one above
What intermediate is involved in the conversion of compound B to compound C?

What intermediate is involved in the conversion of compound B to compound C?

The strong sulfuric acid protonates the hydroxyl group of compound B, resulting in the loss of water as a leaving group and the generation of a carbocation intermediate. Since this carbocation carbon is attached to three other carbons, this is a tertiary carbocation. It is bound to the phenyl substituent, a methyl group, and the branched carbon chain.
The strong sulfuric acid protonates the hydroxyl group of compound B, resulting in the loss of water as a leaving group and the generation of a carbocation intermediate. Since this carbocation carbon is attached to three other carbons, this is a tertiary carbocation. It is bound to the phenyl substituent, a methyl group, and the branched carbon chain.
Compare your answer with the correct one above
Carbon 1:

Carbon 2:

Let's say we react the given compound with  . During the first step of the reaction, will the hydrogen be added to carbon 1 or carbon 2, why?
. During the first step of the reaction, will the hydrogen be added to carbon 1 or carbon 2, why?
Carbon 1:

Carbon 2:

Let's say we react the given compound with 
The correct answer is: carbon 1 because the intermediate will then be a secondary carbocation.
This is a case of  addition across a double bond where
addition across a double bond where  stands for any halide. The first step in this reaction is the attack of
stands for any halide. The first step in this reaction is the attack of  by the double bond. This will create two intermediates, the first being the halide anion
by the double bond. This will create two intermediates, the first being the halide anion  (so in our case
(so in our case  ), the second being a carbocation on our compound at one of the two carbons that formerly shared the double bond.
), the second being a carbocation on our compound at one of the two carbons that formerly shared the double bond.
If the hydrogen attached the carbon 2 then we would have a positive charge on carbon 1 and vice versa. A positive charge on carbon 1 is known as a primary carbocation (a carbon attached to 1 other carbon or function group), which is rarely if ever seen due to its overwhelming instability. A positive charge on carbon 2 is known as a secondary carbocation (a carbon attached to 2 other carbons or functional groups) and is much more stable than a primary carbocation.
We would want the more thermodynamically stable intermediate for our reaction to proceed, so we would want the positive charge on carbon 2 and the hydrogen attached to carbon 1.
The correct answer is: carbon 1 because the intermediate will then be a secondary carbocation.
This is a case of 




If the hydrogen attached the carbon 2 then we would have a positive charge on carbon 1 and vice versa. A positive charge on carbon 1 is known as a primary carbocation (a carbon attached to 1 other carbon or function group), which is rarely if ever seen due to its overwhelming instability. A positive charge on carbon 2 is known as a secondary carbocation (a carbon attached to 2 other carbons or functional groups) and is much more stable than a primary carbocation.
We would want the more thermodynamically stable intermediate for our reaction to proceed, so we would want the positive charge on carbon 2 and the hydrogen attached to carbon 1.
Compare your answer with the correct one above

If the carbon being pointed to was deprotonated (resulting in a positive charge on it). Would the resonance form (the positive charge being redistributed to the carbon with a bromine) be more stable than a secondary carbocation? Why?

If the carbon being pointed to was deprotonated (resulting in a positive charge on it). Would the resonance form (the positive charge being redistributed to the carbon with a bromine) be more stable than a secondary carbocation? Why?
The resonance form of this compound would put the positive charge on the carbon attached to the bromine. Unfortunately this carbon is already slightly positive due to the electron withdrawing effects of bromine due to its high electrophilicity. So this resonance form would be more unstable than a secondary carbocation due to the increased concentration of positive charge from bromine's electron withdrawal.
The resonance form of this compound would put the positive charge on the carbon attached to the bromine. Unfortunately this carbon is already slightly positive due to the electron withdrawing effects of bromine due to its high electrophilicity. So this resonance form would be more unstable than a secondary carbocation due to the increased concentration of positive charge from bromine's electron withdrawal.
Compare your answer with the correct one above
Which of the following compounds would you expect to undergo a nucleophilic addition reaction?
Which of the following compounds would you expect to undergo a nucleophilic addition reaction?
When dealing with carbonyl compounds, remember that a carboxylic acid and all of its derivatives will undergo nucleophilic substitution. Aldehydes and ketones will undergo nucleophilic addition. Propanal is a three-carbon aldehyde, and will thus undergo nucleophilic addition.
Acetic acid is a carboxylic acid, methyl ethanoate is an ether, and ethanamide is an amide; each of these would undergo nucleophilic substitution.
When dealing with carbonyl compounds, remember that a carboxylic acid and all of its derivatives will undergo nucleophilic substitution. Aldehydes and ketones will undergo nucleophilic addition. Propanal is a three-carbon aldehyde, and will thus undergo nucleophilic addition.
Acetic acid is a carboxylic acid, methyl ethanoate is an ether, and ethanamide is an amide; each of these would undergo nucleophilic substitution.
Compare your answer with the correct one above
What is created when a ketone is reacted with a phosphorus ylide?
What is created when a ketone is reacted with a phosphorus ylide?
The Wittig reaction involves a ketone or aldehyde reacting with a phosphorus ylide, a molecule with a negatively charged carbanion. The ketone will undergo nucleophilic addition and form a betaine. This intermediate will then form an alkene with a triphenylphosphine oxide being released. The Wittig reaction will form a mixture of both cis and trans isomers if the carbanion has two different substituents.
Wittig general reaction:

The Wittig reaction involves a ketone or aldehyde reacting with a phosphorus ylide, a molecule with a negatively charged carbanion. The ketone will undergo nucleophilic addition and form a betaine. This intermediate will then form an alkene with a triphenylphosphine oxide being released. The Wittig reaction will form a mixture of both cis and trans isomers if the carbanion has two different substituents.
Wittig general reaction:
Compare your answer with the correct one above