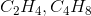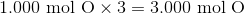Empirical and Molecular Formulas
Help Questions
AP Chemistry › Empirical and Molecular Formulas
What is the empirical formula for a compound that contains 

Explanation
We're given the percent composition of the elements carbon and hydrogen in a given compound, and we're asked to determine the empirical formula.
The first thing we have to do is determine the relative molar ratios of each element. In this case, because we are given the percent composition, we can assume that we're starting off with 
Following that, we need to use the molar mass of each element in order to convert it from grams to moles.
In other words, what we have found is that in our compound, for every 

But remember, the empirical formula for any given compound tells us the lowest whole number ratio of the compound's constituent elements. Therefore, we'll need to divide each value by the lowest number out of all the values available.
What this tells us is that for every 

A molecule with the empirical formula 

Explanation
Start by finding the molar mass of of 
Now, divide the molar mass of the molecule by the molar mass of its empirical formula.
In order to find the molecular formula, you will need to multiply the empirical formula by 

What is the correct molecular formula for phosphoric acid?
Explanation
Phosphoric acid combines a single phosphate ion with three hydrogen ions. The correct molecular formula is 



Vanillin is composed of 


What is the empirical formula of vanillin?
Refer to the following table for the atomic masses of the elements shown:
Explanation
In order to calculate the empirical formula of vanillin from the given percent composition data, we can pretend we have a 100 g sample vanillin and from there estimate how many grams of each element make up the sample. For example, Vanillin is 
After finding the number of moles of each element in the hypothetical 100 g sample, we look at the ratio between the moles of each element by dividing all the samples by smallest number of moles. After finding the ratios of the samples, we are able to use the empirical formula of the vanillin by rounding up to the nearest whole number for each sample. If the ratios are not close enough to round up (like in this example), we multiply the ratios all by the same number until each number is equal to a whole number nicely. See calculations below:
We need whole numbers, so multiply each of the resulting numbers by 3 to get rid of the fractions.
This gives us 
Which pair of formulas represents the empirical formula and molecular formula for a certain compound?
Explanation
To find an empirical formula, take a molecular formula and divide the subscript of each element by the greatest common factor of all the subscripts. In this case, the only pair that works is